Trending Articles of the Week
Trending Articles of the Week
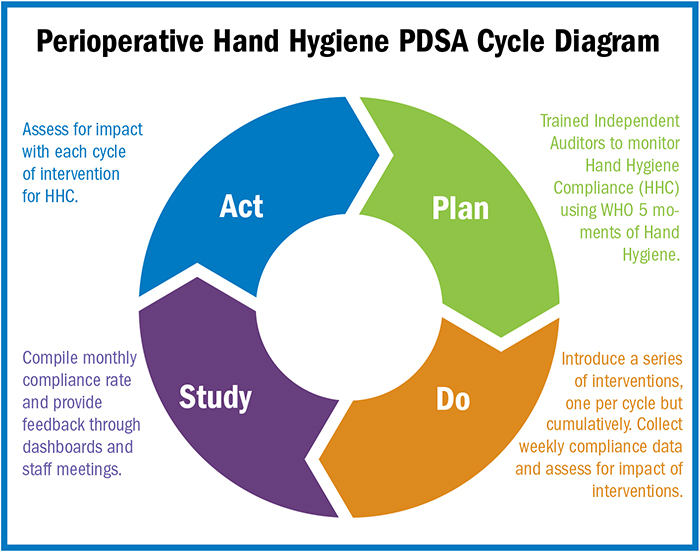
- OR Manager Conference first place poster winner champions hand hygiene among staff
- Capital funding crunch spurs creative hospital financing
- Survey: Dip in satisfaction with little change in compensation
- OSA screening boosts patient safety and bottom line
- Fresh tools, talking points drive sharps safety culture shift
 Exclusive Content
Exclusive Content
- OR Manager Conference second place poster winner highlights assessment tool to mitigate postanesthesia delirium risks
- OR Manager Conference third place poster winner showcases impactful documentation, billing improvements of implants in orthopedic surgery
- OR Manager Conference first place poster winner champions hand hygiene among staff
- Reviewing the evolution of US News’ 2025 best ASCs ranking
- Blast from the past: Best practices for successful implementation of ERAS
 Articles
Articles
- Capital funding crunch spurs creative hospital financing
- 4 ways ASCs balance strict infection control, limited resources
- Reviewing the evolution of US News’ 2025 best ASCs ranking
- Navigating difficult conversations as a perioperative leader
- Welcome to the OR Business Management Conference
Survey: AI reduces administrative burden, improves physician outlook
Physicians are feeling more optimistic about their profession and are beginning to see tangible benefits from AI in reducing administrative tasks, according to the latest Physician Sentiment Survey (PSS) from athenahealth. Physicians’ day-to-day outlook has…
Healthcare employment up in March
Healthcare employment in the US rose by 54,000 from March to April, according to the latest report from the Bureau of Labor Statistics (BLS), released on April 4. That is compared to an overall monthly…
Study: Excessive nurse overtime, agency staffing harm patients
Overreliance on overtime and agency nurse staffing can significantly increase the risk of pressure ulcers and, in the case of agency hours, perioperative hemorrhage or hematoma, according to research published April 2 in JAMA Network…
 Conference Coverage
Conference Coverage
- The Joint Commission launches UNIFY 2025 to advance patient safety, healthcare quality
- OR Manager Conference 2025 agenda announced
- 2025 OR Manager Conference unveils next-level leadership strategies for ASCs and beyond
- Session: Hoisting the Sails—Winning Strategies for Growing Service Lines in an ASC
- Session: Riding the Wave—Considerations to Making Anesthesia Profitable in Today's Labor Market
 Events
Events
 Share your news
Share your news
- Announcing Track Leaders and Committee Members for 2025 OR Manager Conference
- Healthcare providers call for universal platform for recall, other supply disruption alerts
- Nurses projected to see greatest increase in hourly wage by 2033, new study finds
- Sonio, AI-powered prenatal ultrasound management solution, announces implementation by Pediatrix MFM Practice
© Access Intelligence, LLC. All rights reserved. |
Privacy Policy |
Cookie Settings |
Diversity, Equity, Inclusion & Belonging |
Accessibility Statement