 Anesthesiologists
Anesthesiologists
ASA: New research on epidural anesthesia in the OR, reducing SSIs, decreasing overinflation of breathing tubes
Editor's Note On July 14 and 15, the American Society of Anesthesiologists (ASA) hosted a virtual event, the Anesthesia Quality and Patient Safety Meeting, which brought to light new research on when to administer epidural anesthesia in the OR, an anesthesiologist-led infection prevention program reducing rates of surgical site infections…
FDA: Class I recall of Draeger Medical’s Oxylog 3000 Plus Emergency and Transport Ventilators
Editor's Note The Food and Drug Administration (FDA), on July 13, identified the recall by Draeger Medical of its Oxylog 3000 Plus Emergency and Transport Ventilators as Class I, the most serious. The recall was initiated because of reports that the ventilator may not automatically switch back to using AC…
Effect of private insurer bundled payment program for total joints on outcomes
Editor's Note This multi-center study led by Humana Inc, Louisville, Kentucky, finds that a bundled payment program offered by a Medicare Advantage insurer for lower extremity joint replacements was associated with reduced spending without changes in quality. A total of 23,034 lower extremity joint replacement surgical episodes (6,355 bundled, 16,679…
ASA, APSF update recommendations for elective surgery after COVID-19 infection
Editor's Note Because of recent studies, the evolving nature of COVID-19, and widespread vaccination, the American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation (APSF) on June 20 released a joint statement providing updated recommendations for the timing of elective surgery and anesthesia after COVID-19 infection. The recommendations include:…
AI, analytics help match anesthesia supply to surgical demand
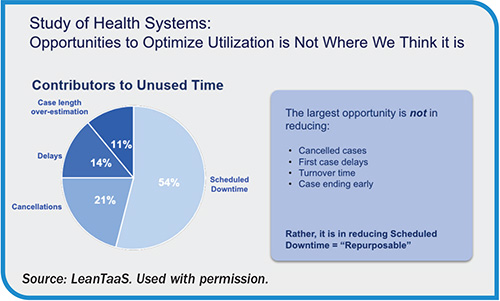
While demand for OR services can be unpredictable, supply is largely fixed—well below the baseline for many hospitals and health systems. The ability to match supply and demand within perioperative services is critical to efficient ORs, as hospitals and health systems must operate with constrained staffing and anesthesia resources. On…
Managing MALS during surgery means addressing chronic pain

Median Arcuate Ligament Syndrome (MALS) presents a challenge to anesthesiologists and surgeons alike in the OR. Clinicians often do not know how to identify and treat this condition, and it can go undetected for years. “I think the reason many anesthesiologists, even pain specialists, aren’t well versed or aware of MALS…
Vanderbilt team develops new protocol for emergency resternotomy
Editor's Note A multidisciplinary cardiovascular intensive care unit (CVICU) team at Vanderbilt University Hospital in Nashville, Tennessee, recently conducted a bedside surgery simulation of a resternotomy to develop a new protocol for the lifesaving procedure, according to the June 15 VUMC Reporter. When a cardiac surgical patient starts bleeding postoperatively…
FDA: Class I recall of Walnut Wearable Smart Thermometers
Editor's Note The Food and Drug Administration, on June 12, identified the recall of the rechargeable Walnut Wearable Smart Thermometer as Class I, the most serious. BearCare, Inc, is recalling the thermometers after receiving reports of skin burns and irritation resulting from use of the device. The company reports five…
FDA letter on supply shortage of nonsterile, single-use pneumatic tourniquet cuffs
Editor's Note In a June 5 letter to healthcare providers, the Food and Drug Administration (FDA) says it is aware of US healthcare facilities and providers experiencing supply constraints of nonsterile, single-use pneumatic tourniquet cuffs. The cuffs are used in elective limb surgeries and in emergency and trauma settings. During…
The Joint Commission revises CAH requirements to align with CMS
Editor's Note The Joint Commission, on May 31, announced that it has added several new and revised elements of performance (EPs) for critical access hospitals (CAHs) that address restraint and seclusion, the complaint process, and unified and integrated structures for CAHs that are part of a multihospital system, effective June…

 Free Daily News
Free Daily News