 Anesthetists
Anesthetists
FDA: Class I recall of Baxter SIGMA Spectrum, Spectrum IQ infusion pumps
Editor's Note The Food and Drug Administration (FDA), on August 1, identified the recall by Baxter Healthcare Corporation of its SIGMA Spectrum Infusion Pump with Master Drug Library (Version 8) and Spectrum IQ Infusion System with Dose IQ Safety Software (Version 9) as Class I, the most serious. The recall…
FDA: Class I recall of GE HealthCare’s TruSignal SpO2 sensors
Editor's Note The Food and Drug Administration (FDA), on July 28, identified the recall by GE HealthCare of its TruSignal SpO2 [arterial oxygen saturation] sensors as Class I, the most serious. The recall was initiated because of issues that may reduce the amount of energy sent to the heart during…
Medical confidentiality breaches on Twitter by anesthesiology, intensive care HCWs
Editor's Note This French study examines the rate of medical confidentiality breaches in tweets by anesthesiology and intensive care healthcare workers (HCWs). Data from 431 tweets with photographs and 9,000 text-only tweets from 1,831 accounts were included in the analysis. Among the findings: There were 44 (10.2%) breaches of medical…
FDA: Class I recall of Draeger Medical’s Oxylog 3000 Plus Emergency and Transport Ventilators
Editor's Note The Food and Drug Administration (FDA), on July 13, identified the recall by Draeger Medical of its Oxylog 3000 Plus Emergency and Transport Ventilators as Class I, the most serious. The recall was initiated because of reports that the ventilator may not automatically switch back to using AC…
ASA, APSF update recommendations for elective surgery after COVID-19 infection
Editor's Note Because of recent studies, the evolving nature of COVID-19, and widespread vaccination, the American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation (APSF) on June 20 released a joint statement providing updated recommendations for the timing of elective surgery and anesthesia after COVID-19 infection. The recommendations include:…
AI, analytics help match anesthesia supply to surgical demand
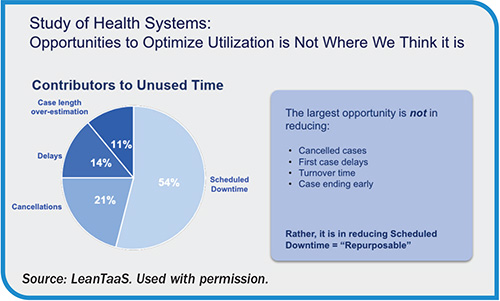
While demand for OR services can be unpredictable, supply is largely fixed—well below the baseline for many hospitals and health systems. The ability to match supply and demand within perioperative services is critical to efficient ORs, as hospitals and health systems must operate with constrained staffing and anesthesia resources. On…
FDA: Class I recall of Draeger Medical’s Seattle PAP Plus, breathing circuit/anesthesia kits
Editor's Note The Food and Drug Administration (FDA), on May 24, identified the recall by Draeger Medical of the Seattle PAP Plus as well as VentStar and other breathing circuit/anesthesia kits as Class I, the most serious. The recall was initiated after finding that glued connections may loosen before or…
Projected growth of nursing, OR-related occupations 2021-2031
Editor's Note According to the Bureau of Labor Statistics, the percent of employment growth from 2021 and projected to 2031 for nursing and operating room-related occupations include: Nurse practitioners—45.7% Physician assistants—27.6% Nurse anesthetists—11.8% Nurse midwives—7.5% Licensed practical and licensed vocational nurses—6.3% Registered nurses—6.2% Surgical technologists—5.9% Nursing assistants—4.7 Surgeons—3.4% Anesthesiologists—1.1%.
SHEA: New SSI guidance recommends antibiotics be discontinued after incision is closed
Editor's Note This update to the 2014 "Strategies to Prevent Surgical Site Infections in Acute Care Hospitals" recommends that antibiotics be discontinued after a patient’s incision has been closed in the OR, even if drains are present. The expert panel members writing the update add that continuing antibiotics after closure…
FDA updates Safety Communication on Halyard surgical N95 respirators
Editor's Note In response to questions, on April 21, the Food and Drug Administration (FDA) provided an update to its Safety Communication that enables the use of existing inventory of the O&M Halyard FLUIDSHIELD Surgical N95 Respirator Masks, Orange (Regular), Level 3, (Model 46727). The FDA recommends that, if necessary,…

 Free Daily News
Free Daily News