Preventing retained items: Time to consider technology?

Technology is starting to take its place as a supplement to manual counts in the effort to prevent retained surgical items (RSIs). RSIs persist despite the emphasis many ORs have placed on tightening their manual counting methods. Recent reports from California are an example of the challenge ORs are up against (sidebar, p 18).
Though rare, RSIs take a heavy toll. Patients with retained items had a rate of death 2.14% higher than controls, excess hospital stays of 2.08 days, and excess costs of $13,315, in a report by Zhan et al. They also increase liability costs.
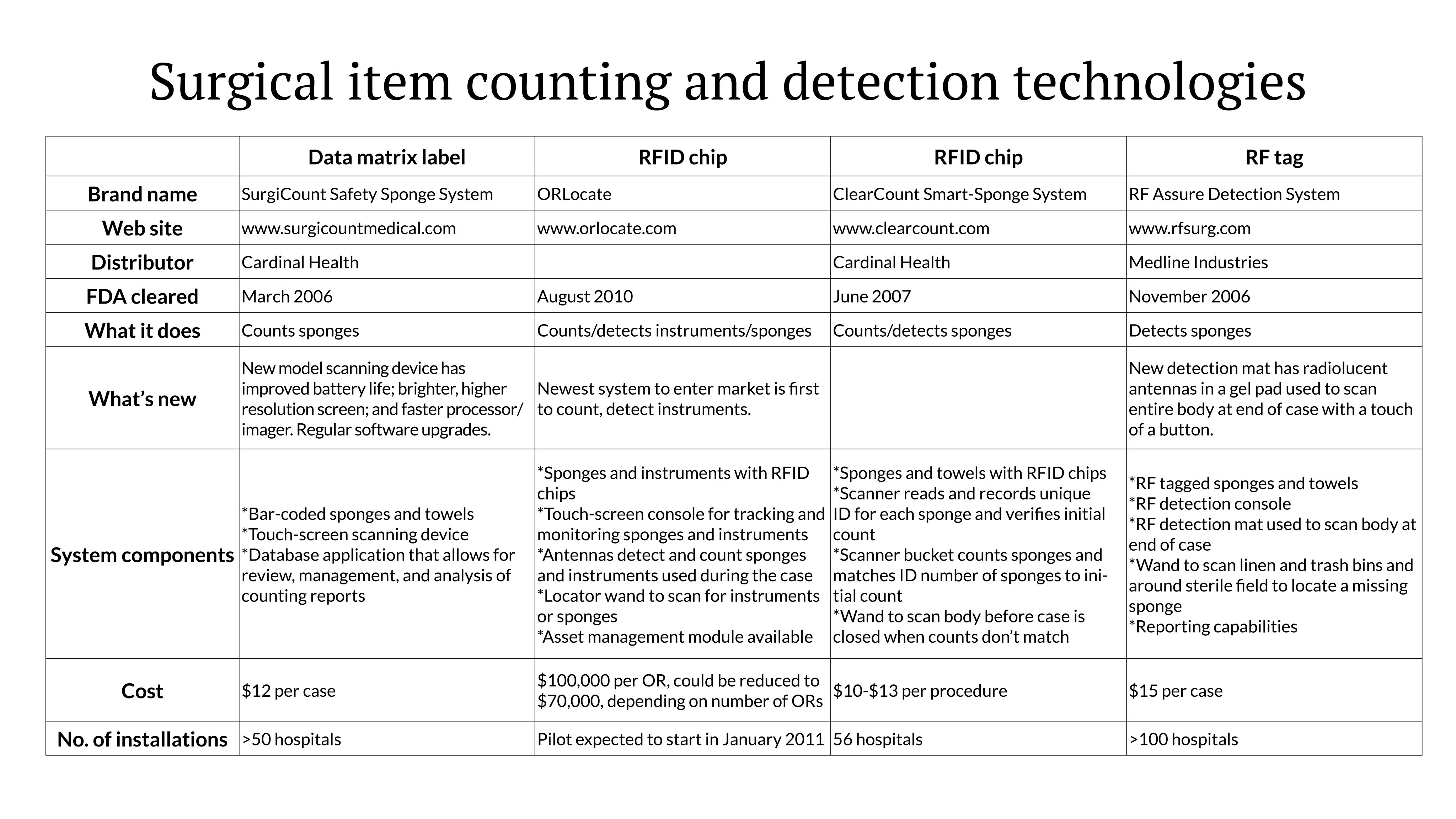
Four companies now offer technologies to help in accounting for instruments and sponges. The newest, ORLocate, which entered the market in August 2010, is the first that can account for instruments. The other companies are upgrading their systems.

A role for technology is being recognized in the literature and professional guidelines.
The Mayo Clinic, examining RSIs that occurred in its organization between 2003 and 2006, concluded that manual counting was unreliable as the primary means for avoiding RSIs and that investigating new technologies for achieving reliable counts is warranted (Cima et al, 2008).
AORN’s new “Recommended practices for the prevention of retained surgical items” say that adjunct technologies may be considered to supplement manual counts, in addition to improving manual counting methods. The recommendations advocate a multidisciplinary approach to accounting for soft goods, sharps, and instruments plus standardized measures for counting and addressing count discrepancies.
An expert on RSI prevention, surgeon Verna Gibbs, MD, advises caution when considering technology.
“Technology adds another layer to already complex OR systems,” she observes. “And technology requires human interface and interaction, which invite new opportunities for error. We also haven’t seen how all the new developments will actually work in OR environments. ”
She says sponges have been retained with the new systems, “because humans operate the technology.
“Technology is not the answer but can assist with a difficult and persistent problem. It is up to hospitals to look at all the solutions that are out there and find what will work best for them.”
Technology is also expensive. Each technology has sponges that are unique to its system and provide the companies with a continuing source of revenue. Once a hospital buys one company’s sponges, it can’t easily change to another system, Dr Gibbs points out.
Dr Gibbs, developer of the NoThing Left Behind campaign for RSI prevention (www.nothingleftbehind.org), is professor of clinical surgery at the University of California, San Francisco, and a surgeon at the San Francisco Veterans Affairs Medical Center. She has been testing a refined manual counting method, called Sponge ACCOUNTING, which she says is coming up on a year of experience in 40 hospitals with no retained items reported.

Here’s an update on the 4 companies that have Food and Drug Administration (FDA) clearance for their counting/detection systems.
The systems perform 3 basic types of functions: count sponges, detect sponges, or count and detect sponges and instruments.
Counting and detecting instruments
As the newest technology, ORLocate (www.orlocate.com) counts, tracks, and monitors both surgical instruments and sponges in the OR using radiofrequency identification (RFID).
Each instrument and sponge is tagged with an RFID chip, giving the item a unique identity that tells not only where the item is but which item it is. The system confirms that counts are correct or incorrect, and if a sponge or instrument is missing, which one it is.
The RFID chip, the size of a small hearing aid battery, comes embedded in sterile surgical sponges and can be laser-welded in a ring to existing instruments. A flat surface area of at least 3 mm is needed to attach the chip.
The system also requires trays with detecting antennas for the Mayo stand and back table as well as a kick bucket with antenna, which can read how many and what kind of sponges or instruments are placed on or in them.
Dr Gibbs terms as “revolutionary” the company’s ability to attach to instruments an RFID chip that can withstand sterilization.
ORLocate’s general manager, Donald Mudd, says a common question the company receives is, “How do you know the chip won’t fall off in the sterilizer?” He says the company’s laboratory testing shows there have been over a thousand cycles of sterilization of these instruments without failures. “The instrument will have to be replaced before the chip,” Mudd says.
The system costs $100,000 per OR, which could be reduced to $70,000, depending on the number of ORs. The company has a mobile lab for retrofitting existing instruments. If a hospital buys 5 OR systems, the company will tag 1,000 instruments at no charge; if 10 systems are purchased, 2,000 instruments will be tagged at no charge. Additional instruments are tagged for a nominal price.
ORLocate expects to pilot its system in January 2011.
Asset management system
ORLocate offers an additional platform for use as an asset management system in sterile processing departments (SPD). Dr Gibbs notes this is a unique attribute of this technology. Because each instrument with a chip has a unique identity, the system can be used to determine which instruments are in which trays and to keep track of instruments needing repair. If an instrument is missing, the system can tell which one. The system can also show how many times an instrument has been used and when it is approaching the end of its life cycle. SPD packing station systems list for $14,500 each; an administrative station lists at $13,500.
Computer-assisted sponge counting
The SurgiCount Safety-Sponge System (www.surgicountmedical.com) is the only system that uses a 2-dimensional data matrix label to count sponges. A computer-assisted scanner records the unique code embedded in each sponge. The size and flexibility of the data matrix code allow it to be embedded even in tiny neuro patties and tonsil sponges, the company says. At the end of a case, the system generates a report of the count.
Sponges are scanned and recorded during initial and final counts. Because each sponge has a unique code, the technology will not allow the same sponge to be counted more than once.
The Mayo Clinic in Rochester, Minnesota, has been using the Safety-Sponge System for the past year with no retained sponges identified.
“We concluded that sponges were our biggest problem, and we wanted to add technology to help with the counting,” said Robert Cima, MD, MA, vice chairman, department of surgery and associate professor of surgery at the Mayo Clinic. Speaking at the Managing Today’s OR Suite Conference in the fall in Orlando, he reported that the technology has reduced total reportable RSIs by nearly 70%.
Notes Dr Gibbs, “We have seen this system being adopted at large single institutions that have a lot of complexity and turnover of residents and nurses.”
RFID chip technology
The SmartSponge System (www.clearcount.com), an RFID-based technology, combines sponge counting and detection. The system reads and records a unique identification (ID) number for each sponge during the initial count and provides a 1-to-1 reconciliation in the final count by matching the ID numbers to sponges. The embedded RFID tags are smaller than a dime.
At the beginning of a case, the nurse passes the sponges over a scanner that counts and reads each sponge’s ID. The system’s LCD screen shows the rolling count.
When surgery is complete, the used sponges are placed in a “smart” bucket that counts each sponge, even if the sponges are stuck together, and the count is displayed on the screen. Because RFID does not require a line of sight between the reader and RFID chips, there is no need to separate sponges or orient the chips in order to scan them, the company says.
“The smart bucket may prove to be a real work saver and safety device for OR nurses, who one day may not have to touch bloody sponges to count them,” Dr Gibbs notes.
When initial and final counts don’t match, a wand is used to scan the body before the incision is closed to detect if a sponge is present. A light on the wand turns red, and an alarm sounds when the sponge is found. The sponge must be retrieved and added to the bucket to reconcile the final count.
A small 2006 study of an investigational device using this technology by Alex Macario, MD, and colleagues found a detection accuracy of 100% for the wand device.
RF tag technology
RF Surgical Systems, Inc (www.rfsurg.com) has added a new detection mattress system to its sponge detection technology, which uses RF tagged sponges. The company continues to offer a detection wand for locating lost sponges that may be in the trash, linen, or elsewhere in the room.
RF Surgical uses passive low-frequency RF tags, which the company says perform better than RFID chips in fluids and blood, dense tissue, and through bone and metal without interfering with OR equipment. The RF tags have a yes-no signal to indicate whether an item is present but do not have a means to count items.
The new detection mattress contains an array of 6 radiolucent antennas. The patient lies on the reusable gel mattress, which is covered with a sheet during surgery. At the end of the case, the nurse pushes a button to perform a hands-free scan of the entire body. If an RF-tagged sponge has been left in the patient, an alarm sounds, and a visual display on the console alerts the staff.
“The mattress eliminates human error in the wanding,” Victoria M. Steelman, PhD, RN, CNOR, FAAN, told OR Manager. Steelman, who is assistant professor in the College of Nursing, University of Iowa Hospital and Clinics, Iowa City, performed a study to find if RF technology could detect sponges through the torso of morbidly obese patients. She found the wand alone had 100% sensitivity if used correctly.
Interim results of a 5-hospital study indicated the RF technology reduced the need for postop x-rays, decreased stress in the OR during closing, and easily identified retained foreign objects. A poster on the study was presented at the American College of Surgeons meeting in Fall 2010 by Christopher Rupp, MD, a surgeon at the University of North Carolina. The study was partly funded by RF Surgical.
References
AORN. Recommended practices for the prevention of retained surgical items. Perioperative Standards and Recommended Practices, 2010 Edition. Denver, CO: AORN.
Cima R R, Kollengode A, Garnatz J, et al. Incidence and characteristics of potential and actual retained foreign object events in surgical patients. J Am Coll Surg. 2008;207:80-87.
Gawande A A, Studdert D M, Orav E J, et al. Risk factors for retained instruments and sponges after surgery. N Engl J Med. 2003;348:229-235.
Gibbs V C, Coakley F D, Reines H D. Preventable errors in the operating room: Retained foreign bodies after surgery. Current Problems Surg. 2007;44:281-337.
Macario A, Morris D, Morris S. Initial clinical evaluation of a handheld device for detecting retained surgical gauze sponges using radiofrequency identification technology. Arch Surg. 2006;141:659-662.
Patterson P. Preventing retained surgical items: What roles does technology play? OR Manager. 2009;25(11):1, 8-11.
Zhan C, Miller M R. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868-1874.
Some retained items beyond reach of technology
Though technology may help prevent some retained items, it wouldn’t have prevented 6 of the 8 events recently reported from California. Eight of the state’s hospitals were fined $25,000 to $75,000 in November 2010 for retained items, including:
- 2 sponges
- A blade for a retractor delivered by a sales rep at the last minute
- Part of a Guidant Heartstring proximal seal system
- A guidewire
- A non-radiopaque blue towel used to stanch bleeding in an emergency case
- A malleable retractor
- A drill bit.
Under California law, retained items are one of 28 medical errors hospitals must report because they place patients at risk of death or serious injury. The state can issue fines of $50,000 for the first event, $75,000 for the second, and $100,000 for the third or subsequent errors at the same hospital.
The reports are posted at www.cdph.ca.gov/Pages/NR10-87-.aspx
5 top reasons counts are likely to fail
An analysis shows the top 5 causes for potential failures involving manual surgical counts are:
- Distraction
- Multitasking
- Not following procedures
- Time pressure
- Emergency cases.
The findings are from a failure modes and effects analysis (FMEA) on managing sponges, conducted by Victoria Steelman, PhD, RN, CNOR, FAAN. The study has been accepted for publication by the AORN Journal.
Education—the number one intervention after a retained item event—won’t fix the problem, she says, because none of the failure modes is related to a knowledge deficit.
Other common strategies used after an event, disciplining the employee or reinforcing the policy, work only 14% of the time, she notes.
Counting is not enough to prevent retained sponges 100% of the time, Steelman notes, and ORs need to start evaluating the available technology for assistance.
“I’m not advocating for one technology over the others,” she says. “I’m just saying that it’s time we start looking at technology to assist us with this. I think all of the technologies are an improvement over counting alone.”