Safety of transoral endoscopic thyroidectomy

Editor's Note The transoral endoscopic thyroidectomy vestibular approach can be safe for select patients, with outcomes similar to those of the open approach, this study finds. Of 425 patients analyzed, the operative time for the transoral approach was longer than the open approach (100.8 vs 79.4 minutes) but transoral patients…
FDA: Class I recall of Datascope/MAQUET intra-aortic balloon pumps
Editor's Note The Food and Drug Administration (FDA) on September 6 categorized the recall by Datascope/MAQUET of its CS100i, CS100, and CS300 intra-aortic balloon pumps as Class I, the most serious. The recall was initiated because of a false blood detection alarm and ingress of fluid into the balloon pump.…
Preadmission frailty predicts adverse outcomes in geriatric trauma patients

Editor's Note In this study, preadmission clinical frailty independently predicted adverse discharge destination (ie, death or discharge to a long-term, chronic or acute care facility) in geriatric trauma patients. The analysis of 266 trauma patients 65 years and older using the Clinical Frailty Scale (CFS) and the laboratory Frailty Index…
FDA: Genetech recalls three lots of Activase
Editor's Note The Food and Drug Administration (FDA) on September 7 announced the recall by Genetech (South San Francisco, California) of three lots of Activase (alteplase) 100 mg vials that were co-packaged with sterile water for injection. The vials of water, manufactured by Hospira Inc (Lake Forest, Illinois), may be…
No consensus on what defines a high-performing system
Editor's Note In this study, researchers reviewed literature from a 10-year period and found no consensus on what defines a high-performing healthcare delivery system or healthcare organization. High performance was variably defined across different dimensions, including: quality (93% of articles) cost (67%) access (35%) equity (26%) patient experience (21%) patient…
FDA: Recall of Hydromorphone HCL and Levophed Injection by Hospira
Editor's Note The Food and Drug Administration (FDA) on September 5 announced the recall by Hospira Inc (Lake Forest, Illinois) of one lot of Hydromorphone HCL injection, USP, CII 2 mg/mL and four lots of Levophed (norepinephrine bitartrate) injection because of a lack of sterility assurance resulting from a damaged…
Causes of malpractice lawsuits against surgical residents
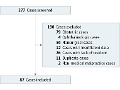
Editor's Note This database review of malpractice lawsuits against surgical residents highlights the importance of perioperative management, particularly among junior residents, and the importance of appropriate supervision by attending physicians as targets for education on litigation prevention. During a 10-year period, 87 malpractice cases involving surgical residents were identified. Results…
CMS grants exceptions for quality reporting to ASCs in Harvey’s path

Editor's Notes The Centers for Medicare & Medicaid Services (CMS) will grant exceptions for quality reporting requirements for ambulatory surgery centers (ASCs) located in the path of Hurricane Harvey, the September 1 ASCA News reports. ASCs in affected counties and parishes in Texas and Louisiana will get exceptions without having…
FDA issues alert for alcohol pads, benzalkonium chloride antiseptic towelettes by Foshan Flying Medical Products
Editor's Note The Food and Drug Administration (FDA) on September 1 issued a Safety Alert notifying healthcare professionals and patients not to use alcohol pads or benzalkonium chloride antiseptic towelettes made by Foshan Flying Medical Products Co Ltd (China) because of lack of sterility and other quality issues. Use of…
Price transparency tool did not reduce spending for California public employees, retirees
Editor's Note Offering a price transparency tool to California public employees and retirees that focused on services such as lab tests, office visits, and advanced imaging services did not lower spending, this study finds. Only 12% of employees used it in the first 15 months after it was introduced, and…
