 Regulations/Legal
Regulations/Legal
Visual cues, education boost hand hygiene compliance
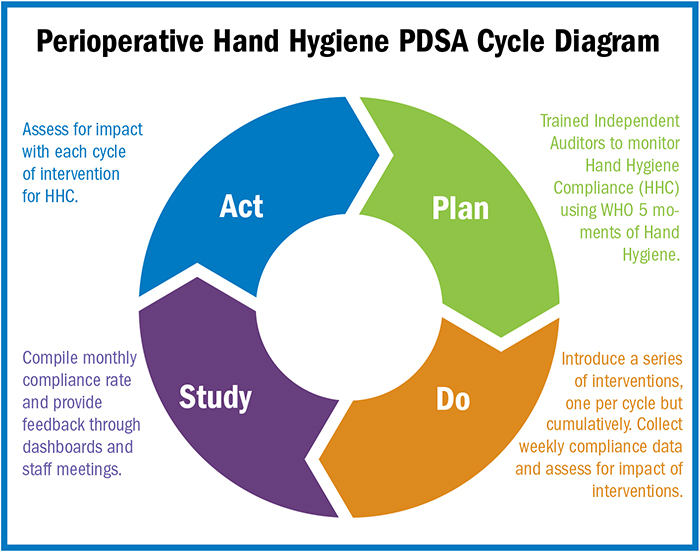
It is often said that small actions lead to big results. This so happens to be the case with hand hygiene compliance (HHC) in healthcare. Imagine a simple act, like washing hands, cutting infection rates by half—hospital-acquired infections (HAIs) and surgical site infections being reduced simply by improving handwashing behaviors.…
FDA announces Class 1 endovascular system, anesthesia system recalls

Editor's Note The US Food and Drug Administration (FDA) designated Getinge’s recall of Vaporizer Sevoflurane Maquet Filling and Sevoflurane Quick-Fil and Philips’ recall of Tack Endovascular Systems as Class 1s, the most severe category indicating serious risk of injury or death. The Getinge recall is an expansion of a 2024…
Cost-shifting concerns rise as GOP eyes Medicaid cuts

Editor's Note Large employers are warning hospitals they will not absorb higher costs if plans by Republicans and the Trump administration for deep Medicaid cuts proceed, a February 28 article in Modern Healthcare reports. The threat of reduced Medicaid funding has reignited concerns about hospitals shifting costs onto employers and…
Tenet Healthcare's strategic ASC expansion, hospital divestitures boost 2024 Q4 results

Editor's Note Tenet Healthcare closed 2024 on a high note, reporting robust fourth-quarter results driven by strategic growth in ambulatory surgery centers (ASCs) and effective cost management, Healthcare Finance February 18 and Healthcare Dive February 13 report. Partnering with United Surgical Partners International (USPI), one of the largest ASC platforms…
Trump signs executive order to enforce hospital price transparency rules

Editor's Note President Donald Trump signed an executive order to enforce healthcare price transparency regulations, according to a February 25 report in Fierce Healthcare. As detailed in the article, the order directs the Departments of Treasury, Labor, and Health and Human Services (HHS) to rapidly implement and enforce price transparency…
Providence Health strike ends with wage increases, staffing changes

Editor's Note Nearly 5,000 healthcare workers at Providence Health in Oregon secured substantial wage increases and improved staffing plans, ending a historic six-week strike that began January 10, according to a February 25 article in MedPage Today. According to the article, the strike involved eight RN bargaining units and marked…
FDA designates Class 1 recall for pacemakers

Editor’s Note The US Food and Drug Administration (FDA) has designated Boston Scientific Corporation’s recall of Accolade Pacemaker devices a Class 1, the most severe category indicating serious risk of injury or death. According to the agency’s February 21 announcement, the recall was motivated by a manufacturing issue that could…
Chinese medical devices threaten US healthcare cybersecurity

Editor’s Note Backdoors in Chinese-made medical monitors could put patients at risk and compromise hospital networks across the US, according to security agencies quoted in a February 23 report from CNBC. The article cites the popular Contec CMS8000 patient monitor as an example. Both the US Food and Drug Administration…
Healthcare M&A faces tighter review as FTC retains guidelines

Editor’s Note In a setback for healthcare mergers and acquisitions (M&A), the Federal Trade Commission (FTC) will continue enforcing strict antitrust guidelines established under the Biden administration, Healthcare Dive February 19 reports. Bucking expectations of a more lenient approach under the new administration, FTC Chair Andrew Ferguson confirmed the decision,…
Healthcare giants prioritize shareholder payouts over patient care

Editor’s Note Shareholder payouts by publicly traded healthcare companies have surged 315% since 2001, raising questions about financial priorities in the sector, according to a February 19 article in Healthcare Finance. A research letter published in JAMA Internal Medicine and co-authored by Yale University researchers found that major healthcare corporations…

 Free Daily News
Free Daily News