 Regulations/Legal
Regulations/Legal
Study: Federal antitrust action minimal relative to number of hospital mergers

Editor's Note Federal regulation of hospital mergers is inadequate, according to an April antitrust enforcement study scheduled to be published by the American Economic Association. According to a June 14 report in Modern Healthcare, researchers at universities including Harvard and Yale analyzed insurance claims data from Aetna, Humana, and UnitedHealthcare,…
FBI, HHS issue healthcare cybersecurity warning

Editor's Note A June 24 advisory from the FBI and Department of Health and Human Services warns healthcare organizations about attempts to steal payments through phishing and social engineering tactics, according to a post from the American Hospital Association (AHA). The attackers target employee email accounts to access login information…
Russian ransomware group threatens cybersecurity beyond London attack

Editor's Note Qilin, a ransomware group based in Russia, claimed responsibility for a cyberattack against pathology services provider Synnovis that paralyzed London Hospitals and is now requesting $50 million, Becker’s Health IT reported June 20. Citing a report from Bloomberg, the article notes that the attack disrupted services at London-based hospitals…
Change Healthcare issues notifications of patient data stolen in cyberattack

Editor's Note Change Healthcare has started to notify health care providers about patient data stolen in the February cyberattack and announced plans to mail affected individuals as well. A unit of UnitedHealth Group, the organization issued the update June 20. “CHC is providing this notice now to help individuals understand…
Ambulatory endoscopy management strategies keep patients, finances healthy
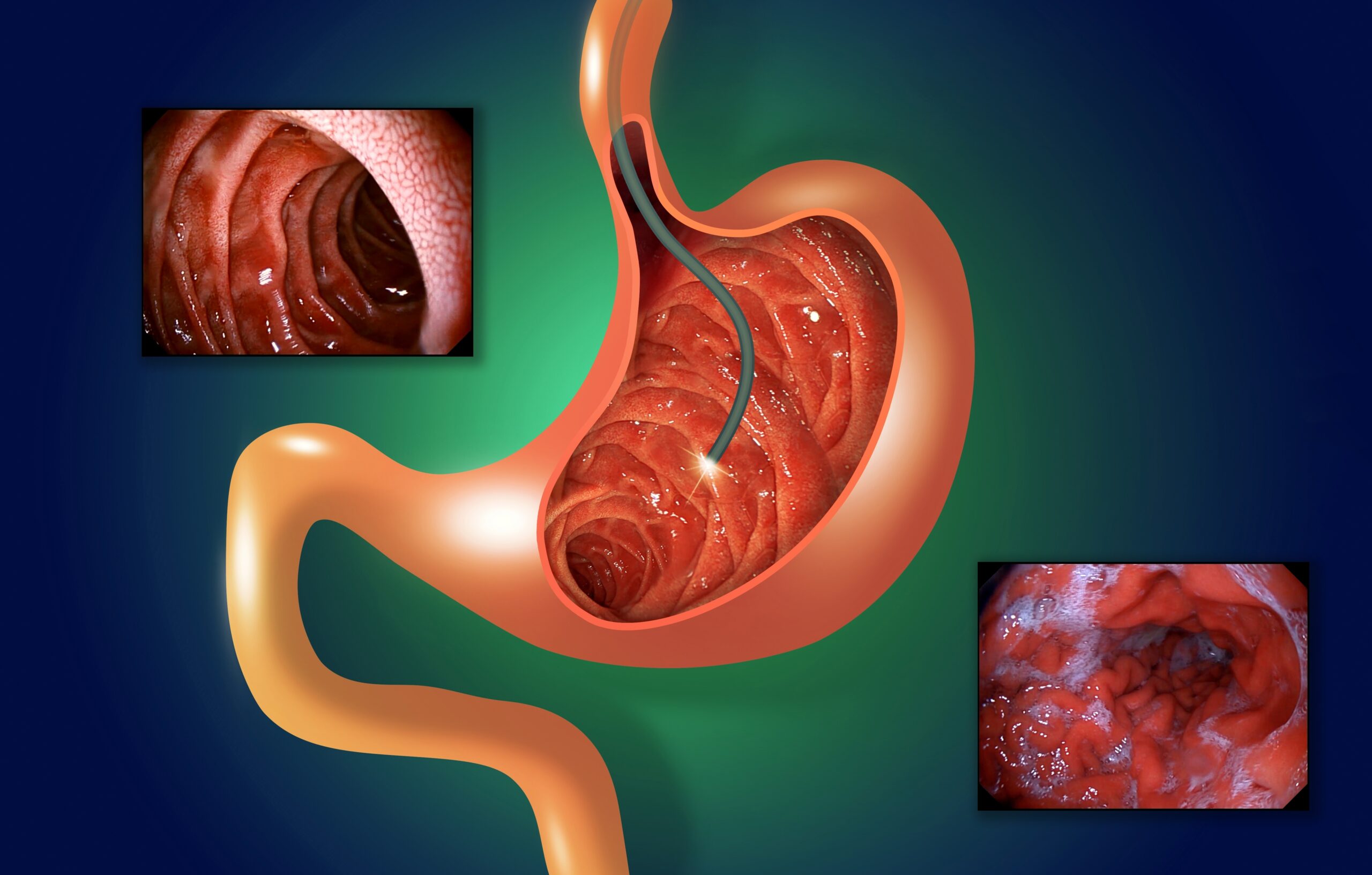
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…
How competency assessment could extend beyond licensing
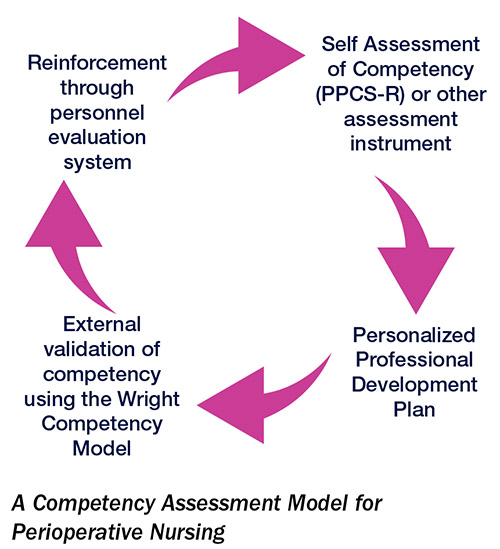
Competency assessment in perioperative nursing—and American healthcare in general—is a story of unrealized potential. Particularly in the wake of the pandemic, staffing shortfalls and financial pressures have made focusing on staff development difficult for nurse leaders. Nonetheless, the argument for investing more in professional development and competency has never been…
Rural hospitals contend with challenging opportunities

Rural hospitals in the US have been facing a prolonged, multifaceted crisis. The literature presents several reasons for why healthcare facilities in rural areas struggle, including shrinking budgets, rising chronic illness and public health issues like addiction and obesity, poor telehealth and broadband access, aging populations, deteriorating mental health, and…
Scaling standards from sterile processing department to clinic
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…
CMS to end Change Healthcare cyberattack assistance program

Editor's Note The Centers for Medicare & Medicaid Services (CMS) has announced assistance for providers affected by the Change Healthcare cyberattack ends next month. According to the June 17 announcement, payments under the Accelerated and Advance Payment (AAP) Program for the Change Healthcare/Optum Payment Disruption (CHOPD) will end July 12,…
Understanding bipartisan push for site-neutral Medicare payments aiming to curb healthcare costs, consolidation

Editor's Note Amid growing concerns over healthcare spending and affordability, there is bipartisan interest in aligning Medicare payments for outpatient services across various care settings through "site-neutral" payments, KFF June 14 reports. As a June 2023 Modern Healthcare article explains, last year Congress reviewed legislation to expand site-neutral payment policies,…

 Free Daily News
Free Daily News