 Regulations/Legal
Regulations/Legal
Postpandemic applicability, usage of UV disinfection technology

The COVID-19 pandemic highlighted the importance of proper disinfection to manage and contain the spread of the disease. During the pandemic, ultraviolet (UV) disinfection technology played an important role in safeguarding public health and combatting COVID-19. Various uses of UV technology have become available on the market and can be…
Minimizing wasted 'In-OR' minutes at start of day at a pediatric AMC
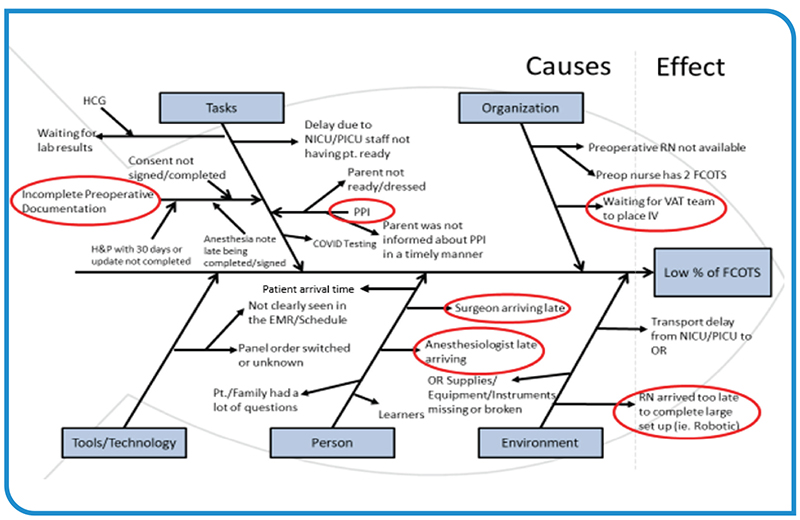
The OR is the financial motor of a hospital. As such, inefficiencies in this space adversely impact patient safety as well as the financial stability of the hospital and/or healthcare system. The first case on-time starts (FCOTS) metric is one of the primary benchmarks for assessing OR efficiency. The following…
AAMI: Time, temperature, humidity affecting cleanliness of instruments, medical devices
Editor's Note Researchers for a long time have expressed concern about how time and temperature might contribute to changes that affect the cleaning and sterilization of instruments. However, there have been few studies examining these claims. A September 2023 study in Biomedical Instrumentation and Technology from the Association for the…
Assistance program leads to major drop in ER visits, costs for immigrants
Editor's Note A study from economists and public health officials in the September 2023 issue of the journal American Economic Review: Insights found that when undocumented immigrants were provided assistance to visit primary care doctors via a pilot program, it resulted in a 21% drop in emergency room (ER) use.…
ASC luncheon: Quality Measure Reporting Updates for ASCs
Editor’s Note In this luncheon presentation, OR Manager Conference attendees delved into the Centers for Medicare & Medicaid Services Quality Measure Reporting Program for ambulatory surgery centers (ASCs). Gina Throneberry, MBA, RN, CASC, CNOR, director of education and clinical affairs at the Ambulatory Surgery Center Association (ASCA), shared which quality…
The Joint Commission announces new Speak UP campaign on preventive care
Editor's Note The Joint Commission, on September 13, announced its new Speak Up campaign To Prevent Serious Illness. The campaign is designed to educate patients on finding preventive care services, getting past barriers, and trying to avoid reaching a crisis point with their health. Preventive care includes blood tests, cancer…
The Joint Commission: World Patient Safety Day is September 17
Editor's Note On September 17, World Patient Safety Day, The Joint Commission is partnering with the World Health Organization in recognizing the shared commitment to safe, equitable, high quality care for all and asks all accredited organizations and partners to join in the recognition. This year’s theme, “Engaging patients for…
Effect of Medicaid expansion on reductions in preventable hospitalizations
Editor's Note This study, led by researchers at Wake Forest University School of Medicine, Winston-Salem, North Carolina, examines whether Affordable Care Act Medicaid expansion among Black, Hispanic, and White patients led to reductions in preventable hospitalizations. Data on census population and hospitalizations for ambulatory care sensitive conditions from 2010 to…
ACS addresses opioid prescription misuse with new QI project
Editor's Note The American College of Surgeons (ACS), on September 12, announced a new quality improvement (QI) project that will evaluate the most effective ways to help patients safely manage postoperative pain and reduce the risk of opioid dependence. The project will be conducted in collaboration with Health Care Service…
FDA: Class I recall of Mallinckrodt One-Way Valve, 22F x 22M
Editor's Note The Food and Drug Administration (FDA), on September 12, identified the recall by Mallinckrodt Manufacturing of its One-Way Valve, 22F x 22M, as Class I, the most serious. The recall was issued because the devices are not opening properly, which prevents or reduces the flow of ventilated air…

 Free Daily News
Free Daily News