 Regulations/Legal
Regulations/Legal
Supreme Court case could threaten colorectal cancer screening access, ACG warns

Editor's Note The American College of Gastroenterology (ACG) is warning that millions of Americans could lose access to essential colorectal cancer (CRC) screenings if the Supreme Court rules against the role of the US Preventive Services Task Force (USPSTF) in guiding preventive care coverage. According to the organization’s March 7…
Study: Implicit bias linked to low-value vascular procedures, worse outcomes for Black patients

Editor's Note Reducing the negative influence of implicit bias requires system-level interventions to ensure procedures align with best practices for all patients, according to results of new research on outcomes for vascular surgery patients. Published February 26 in JAMA Surgery, the study showed that implicit racial bias among vascular specialists…
New legal standard redefines medical negligence

Editor's Note A revised legal standard for assessing medical negligence in the US shifts away from customary medical practice and toward a more patient-centered definition of reasonable care, according to a February 26 letter published in Jama Network. Following a 2024 update from the American Law Institute, the new framework…
Infusion pumps deemed high-risk in FDA early alert

Editor's Note Baxter Healthcare Corporation has issued a letter to affected customers recommending certain Spectrum infusion pumps be removed from where they are used or sold, according to a March 5 early alert from the US Food and Drug Administration (FDA). The FDA notice concerns the Sigma Spectrum Infusion System…
AAAHC seeks stakeholder feedback on revised v44 standards to elevate patient-centered care

Editor's Note The Accreditation Association for Ambulatory Health Care (AAAHC) has opened a public comment period for proposed revisions to its v44 standards, ASC Focus February 28 reports. From now until March 29, healthcare professionals and other stakeholders are encouraged to share insights that will help refine the guidance for…
Smart segregation, storage reduce biohazardous waste risks

From sharps and blood-soaked surgical instruments to discarded anesthetic agents, biohazardous waste from ORs can threaten human health and the environment. In addition, failure to adhere to regulatory requirements can result in significant fines. However, the volume and diversity of biohazardous waste can create challenges with managing this material. Mitigating…
Fresh tools, talking points drive sharps safety culture shift
Takeaways • The prevalence of needlesticks and other sharp object injuries to OR team members is 42.8%, an increase of 16% over the past decade. • New research and perspectives are shaping the discourse around sharps safety, such as new and expanded evidence-based practices presented in AORN’s 2025 update to…
Visual cues, education boost hand hygiene compliance
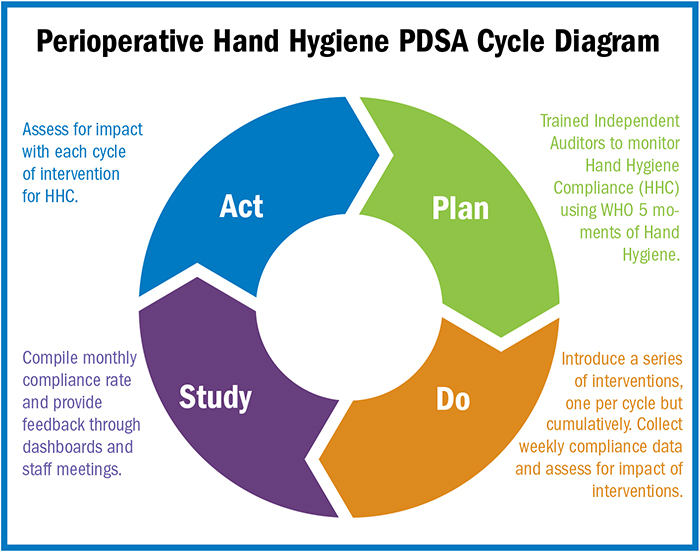
It is often said that small actions lead to big results. This so happens to be the case with hand hygiene compliance (HHC) in healthcare. Imagine a simple act, like washing hands, cutting infection rates by half—hospital-acquired infections (HAIs) and surgical site infections being reduced simply by improving handwashing behaviors.…
FDA announces Class 1 endovascular system, anesthesia system recalls

Editor's Note The US Food and Drug Administration (FDA) designated Getinge’s recall of Vaporizer Sevoflurane Maquet Filling and Sevoflurane Quick-Fil and Philips’ recall of Tack Endovascular Systems as Class 1s, the most severe category indicating serious risk of injury or death. The Getinge recall is an expansion of a 2024…
Cost-shifting concerns rise as GOP eyes Medicaid cuts

Editor's Note Large employers are warning hospitals they will not absorb higher costs if plans by Republicans and the Trump administration for deep Medicaid cuts proceed, a February 28 article in Modern Healthcare reports. The threat of reduced Medicaid funding has reignited concerns about hospitals shifting costs onto employers and…

 Free Daily News
Free Daily News