 Safety/Quality
Safety/Quality
Report shows alarming trends in nurse engagement: Burnout, generational differences highlighted
Editor's Note A September 2023 report from market research and consulting company PRC and published by Nursing Academy looked at retention, burnout, and engagement issues among more than 1,900 nurses at 37 hospitals. The report found that only 45% of nurses report being “fully engaged” at work, while 14.1% percent…
Comparing COVID-19 booster shots–and whether to wait
Editor's Note With COVID-19 rates rising again, and winter cold and flu season around the corner, it is time for healthcare personnel to get a COVID-19 booster shot to help protect against illness–and recommend patients to do the same. In a September 2023 TIME Magazine article, doctors weigh in on…
Inflammation following abdominal surgery key marker for adverse outcomes
Editor's Note In this retrospective cohort study, researchers looked at the association between inflammation and various outcomes following major abdominal surgery. The study, titled "Postoperative systemic inflammation after major abdominal surgery: patient-centered outcomes," was published in the journal Anaesthesia, of the Association of Anaesthetists, in August 2023. Data came from…
New AI tool matches human precision in assessing surgical margins for breast cancer
Editor's Note Researchers from the University of North Carolina developed an artificial intelligence (AI) model that can predict whether cancerous tissue has been fully removed from the body during breast cancer surgical procedures. Their findings were published in the Annals of Surgical Oncology August 2023 issue. During the procedure, surgeons…
Addressing mistreatment of transgender people in healthcare encounters
Editor's Note Transgender people are subject to mistreatment in healthcare encounters, including harassment, assault, and denial of care, according to this September 2023 qualitative study published by Annals of Family Medicine. This study comprised 30 transgender adults and found the following experiences among transgender patients: Transgender people often found clinicians’…
Leapfrog Group recognizing hospitals that excel in diabetes care
Editor's Note The nonprofit Leapfrog Group has joined forces with the American Diabetes Association (ADA) to recognize hospitals providing exceptional care for patients with diabetes, Chief Healthcare Executive September 12 reports. Earning the designation will be based on how well hospitals meet ADA’s standards of care, in addition to their…
Minimizing wasted 'In-OR' minutes at start of day at a pediatric AMC
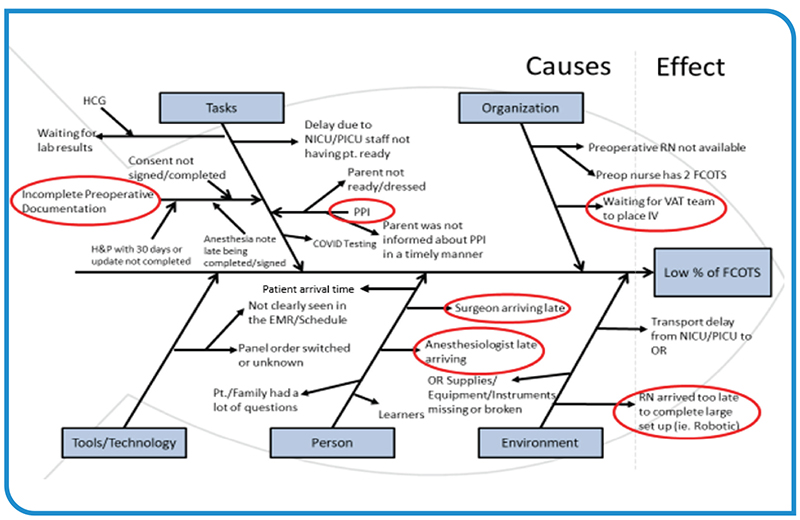
The OR is the financial motor of a hospital. As such, inefficiencies in this space adversely impact patient safety as well as the financial stability of the hospital and/or healthcare system. The first case on-time starts (FCOTS) metric is one of the primary benchmarks for assessing OR efficiency. The following…
Study shows serious downside, risks to postop opioid prescription at discharge
Editor's Note This cohort study, published by the British Journal of Anesthesia in September 2023, finds that surgical patients who are discharged with prescribed opioids have higher risks of hospital readmission and mortality. The study looked at data from a territory-wide retrospective cohort of patients in Hong Kong who underwent…
AAMI: Time, temperature, humidity affecting cleanliness of instruments, medical devices
Editor's Note Researchers for a long time have expressed concern about how time and temperature might contribute to changes that affect the cleaning and sterilization of instruments. However, there have been few studies examining these claims. A September 2023 study in Biomedical Instrumentation and Technology from the Association for the…
Long nursing shifts in mental health wards lead to increased patient incidents
Editor's Note A new study in Journal of Nursing Management, published on September 6, found that when the majority of nursing shifts in mental health and community wards were 12 hours or longer, there was a significant increase in the risk of patient incidents. Some highlights of the study include:…

 Free Daily News
Free Daily News