 Safety/Quality
Safety/Quality
Association of COVID-19 with incidence of HAIs in California hospitals
Editor's Note This study, by researchers at the University of California, San Francisco, and California Department of Public Health, finds significant increases in healthcare-associated infections (HAIs) in California acute-care hospitals during the COVID-19 pandemic, specifically, central-line-associated bloodstream infections (CLABSIs) and methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections (BSIs). The researchers found…
FDA: Class I recall of Baxter Hillrom WatchCare IMS for RF interference risk
Editor's Note The Food and Drug Administration, on November 23, identified the recall by Baxter Hillrom of its WatchCare Incontinence Management System (IMS) as Class I, the most serious. The recall was initiated because of the risk for radiofrequency (RF) emissions from WatchCare devices that may interfere with other devices,…
Antiviral, antibiotic shortages cause strain on healthcare providers
Editor's Note A shortage of medications used in treatment of pediatric illnesses are causing additional challenges for healthcare providers amid spikes in respiratory viruses, according to a November 22 CNN story, reports Becker’s Hospital Review November 23. It was reported by the American Society of Health-System Pharmacists (ASHP) that as…
Part 1 of Benchmark Questionnaire on OR Business Practices closes December 5, 2022
Editor's Note Part 1 of the Benchmark Questionnaire on Operating Room Business Practices (Elective Cases Only) closes December 5, 2022 at 8 pm ET. We ask that you take 10 minutes to complete the survey to help highlight best OR Practices for our readers. The first part of this 2-part survey…
Parents’ COVID-19 vaccination status linked to children's hospital admissions
Editor's Note This French study finds that during the Delta and Omicron periods of COVID-19, parents’ vaccination status was associated with hospital admissions for their children younger than 5 years of age, for whom vaccines were not licensed. During the study period, 599 children were admitted to the hospital. For…
FDA: Class I recall of Insulet Omnipod DASH PDM
Editor's Note The Food and Drug Administration (FDA) on November 16 identified the recall by Insulet of its Omnipod DASH Insulin Management System Personal Diabetes Manager (PDM) as Class I, the most serious. The recall was initiated because of PDM battery issues, including: Battery swelling Fluid leakage from the battery…
Augmented and virtual reality bring high-tech efficiency to the OR

COVID-19 was a catalyst that helped accelerate innovation in healthcare by a decade, says Jay Banerjee, chief operating officer and cofounder of ImmersiveTouch, a virtual reality (VR) surgery company in Chicago. “Many things that would have taken 20 to 30 years happened sooner,” Banerjee says. “Telemedicine; electronic health records; remote…
Editorial

After writing my latest article on surgical robots (Operationalizing a robotics program for evenings, weekends, pp 13–15, 19) for this issue of OR Manager, I reminisced on how the surgical robot has evolved and how my interest in surgical robots began. Reminiscing about robots It all began with the…
A success story: Coordination, teamwork, and collaboration in aerodigestive procedures

The Ann & Robert H. Lurie Children’s Hospital in Chicago has a mission: to successfully serve children with complex airway disorders requiring pulmonary, upper digestive tract, sleep, voice, and swallowing evaluations. As a 360-bed facility ranked among the country’s top children’s hospitals by US News & World Report and housing more than 1,800…
Operationalizing a robotics program for evenings, weekends
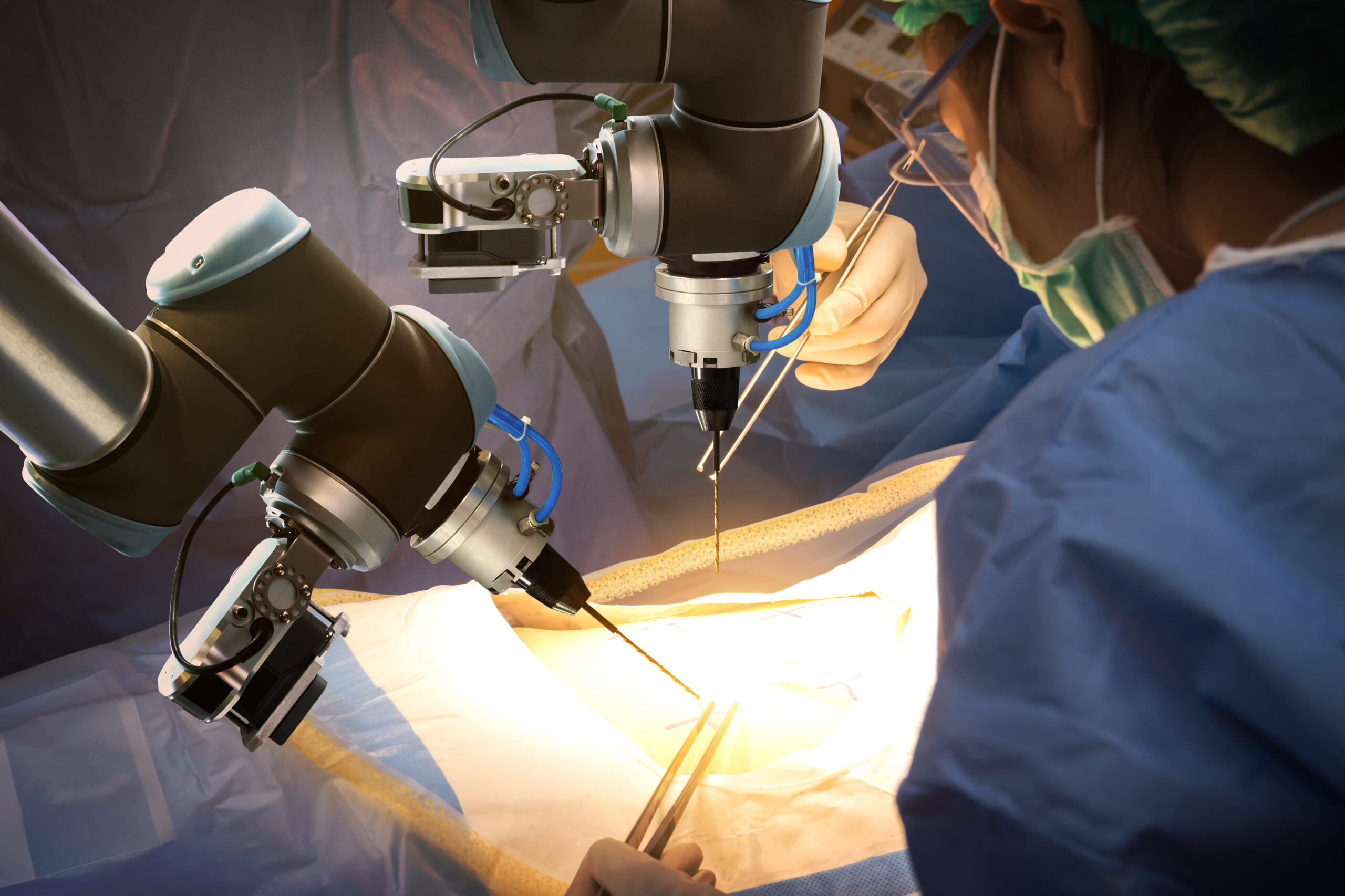
The St Elizabeth Healthcare organization in northern Kentucky has eight facilities, including two surgery centers. The flagship hospital on the Edgewood campus has 534 beds and 22 ORs and typically performs some 14,000 surgical procedures per year. Robots help manage the volume. The Edgewood, Florence, and Ft Thomas hospitals have…

 Free Daily News
Free Daily News