 Sterilization & Disinfection
Sterilization & Disinfection
Economic benefits of low-temperature vs steam sterilization of endoscopes
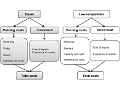
Editor's Note This Australian study finds it is a good economic decision for large healthcare facilities to invest in low-temperature systems for sterilization of steam-sterilizable endoscopes. Increased costs associated with low-temperature systems were outweighed by savings from fewer instrument repairs. Based on their calculations, the researchers estimated a savings of…
Alcohol fixation of bacteria to instruments increases cleaning difficulty

Editor's Note Treating contaminated surgical instruments with alcohol, allowing them to dry, or allowing them to soak in water for extended periods of time increases cleaning difficulty and may contribute to sterilization inefficacy, this study finds. Soaking or spraying instruments with alcohol significantly reduced viable bacterial numbers, but significantly increased…
Joint Commission: HLD, sterilization top challenges for ambulatory health care

Editor's Note Leading the Joint Commission’s Top Ten Challenging Standards for accredited ambulatory health care organizations during the past year was infection control standard IC.02.02.01, EP 2: The organization reduces the risk of infections associated with medical equipment, devices, and supplies by performing a high-level disinfection and sterilization. In 2016,…
Study: Ureteroscopes remain contaminated after cleaning, sterilization

Editor's Note Techniques used to clean and sterilize flexible ureteroscopes left behind contamination that included debris, residue, and bacteria, in this study presented June 14 at the 44th Annual Conference of the Association for Professionals in Infection Control. Researchers with Ofstead & Associates (St Paul, Minnesota) sampled 16 ureteroscopes at…
FDA releases list of reusable devices requiring new validated IFU, validation data
Editor's Note The Food and Drug Administration (FDA) on June 9 released a list of reusable devices that will require new validated instructions for use (IFU) and validation data in premarket notifications regarding cleaning, disinfection, and sterilization. These actions are effective August 8, 2017. The list includes: Bronchoscopes (flexible or…
Joint Commission: Safety update on improperly sterilized, HLD equipment

Editor's Note The Joint Commission on May 24 issued a new Quick Safety update on improperly sterilized or high-level disinfected (HLD) equipment, which continues to be a frequently scored noncompliant standard (IC 02.02.01). The update includes expanded safety actions to help leaders oversee sterilization and HLD processes and ensure that…
Consider a sterilization subject matter expert to increase compliance
Compliance with sterilization and disinfection standards is critical to patient safety, yet Joint Commission surveyors continue to cite healthcare facilities for noncompliance with high-level disinfection (HLD) and sterilization processes during accreditation surveys. Organizations with HLD and/or sterilization breaches during a survey may receive an immediate threat to life or adverse…
Olympus redesigned duodenoscope linked to new infection outbreak

Editor's Note A new outbreak of infections outside the US have been tied to a duodenoscope Olympus modified last year to reduce the risk of transmitting bacteria between patients, the March 22 Los Angeles Times reports. The outbreak of Klebsiella pneumoniae in five patients occurred at the end of December…
Arm your staff with strategies to prevent HAIs
Each year, more than 700,000 patients in acute care hospitals fall prey to healthcare-acquired infections (HAIs), and approximately 75,000 hospitalized patients die from them. Such statistics have gotten the attention of regulatory agencies that are determined to reduce these numbers. The Healthcare-Acquired Infections and Medical Technology Stakeholder Event held in…
Rigorous reprocessing doesn’t free scopes of contamination
Editor's Note This study by Cori L. Ofstead, MSPH, and associates found that more rigorous reprocessing was not consistently effective in freeing endoscopes of contamination, and many had scratches and dents that could harbor blood, tissue, and bacteria. Even after reprocessing using current guidelines or additional measures, 12 of 20…

 Free Daily News
Free Daily News