 Supply Chain/Technology
Supply Chain/Technology
ECRI Institute announces healthcare supply chain award winners
Editor's Note The ECRI Institute on April 23 announced the winners of its seventh annual Healthcare Supply Chain Achievement Award, which recognizes hospitals and healthcare systems for their comprehensive and effective use of ECRI Institute’s PriceGuide™ and SELECTplus® supply and capital procurement advisory programs. Eleven winners were selected from nearly…
ECRI Institute releases ‘Top 10 Hospital C-suite Watch List’
Editor's Note The ECRI Institute on January 22 announced the released its annual “Top 10 Hospital C-suite Watch List." Among the new and emerging innovations to watch are: Acuity-adaptable rooms, where hospitals keep a patient in the same room from admission to discharge, regardless of acuity level. Insertable cardiac monitors…
New ECRI Institute resource provides solutions for IV-minibag shortage
Editor's Note The ECRI Institute on January 19 announced the release of a special Health Devices guidance article featuring recommendations and alternative solutions for dealing with the IV-fluid minibag (50-250 mL) shortage that resulted form Hurricane Maria in Puerto Rico. The guidance provides a functional equivalence chart for IV solution…
FDA issues guidance on 3D printing of medical devices
Editor's Note Food and Drug Administration (FDA) commissioner Scott Gottlieb, MD, issued a statement December 4 on the FDA’s final guidance on medical device additive manufacturing, also known as 3D printing. The FDA has reviewed more than 100 devices currently on the market that were manufactured on 3D printers, including…
San Antonio to host 2018 OR Business Management Conference
With each passing year, the business of healthcare delivery grows more complex—never more so than in 2017, when a new administration began to grapple with a major overhaul of the Affordable Care Act and changes to provider payment models. Thanks to an unprecedented number of proposals submitted for the 2018…
RTI Surgical announces 3D-printed cervical interbody device
Editor's Note RTI Surgical (Alachua, Florida) on October 25 launched its Fortilink-C interbody fusion system with Tetrafuse 3D technology, the October 26 Becker’s Spine Review reports. Tetrafuse 3D technology combines titanium, allograft bone, and PEEK all in one device. The 3D-printed, polymer-based, cervical interbody device incorporates micro, macro, and nano-rough…
New app helps protect patients’ brains during surgery
Editor's Note A new app developed by students at the University of Wisconsin-Milwaukee’s (UWM’s) App Brewery and a neuropsychologist at the Medical College of Wisconsin (MCW) monitors critical brain functions during neurosurgical procedures in which patients are awake. The app, called “NeruoMapper,” assists neurosurgeons who are removing tumors by helping…
Two firms awarded grants to develop medical devices for children
Editor's Note The Philadelphia Pediatric Medical Device Consortium (PPMDC) has chosen two companies out of eight finalists to receive grants of $50,000 each to develop medical devices for children. The devices include a: speech generating system that allows hospitalized children who are unable to speak to communicate with clinicians handheld…
Surgeons perform first magnetic compression anastomosis (magnamosis) in humans
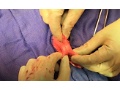
Editor's Note In this pilot trial, surgeons used a pair of magnets (ie, Harrison rings) to create an intestinal anastomosis without sutures or staples in five patients. For each procedure, one Harrison ring was placed in the lumen of each intestinal segment, and then the rings were brought together and…
Scientists create artificial heart with 3D printing

Editor's Note Researchers from Zurich, Switzerland, have created an artificial heart out of silicone using 3D printing. The artificial heart, which mimics the human heart in shape and function, presently has a lifespan of approximately 3,000 beats, or about 30 to 45 minutes. The researchers hope to develop a more…

 Free Daily News
Free Daily News