 Supply Chain/Technology
Supply Chain/Technology
AAMI updates sterilization materials compatibility guidance

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has released the updated AAMI TIR17:2024; Compatibility of Materials Subject to Sterilization, its first revision since 2017. This guidance provides essential information for medical device manufacturers, designers, and sterilization professionals on how sterilization methods impact materials and packaging. Updates…
Health system to pilot AI-powered OR cameras; leaders acknowledge privacy challenges
Editor's Note UC San Diego Health plans to pilot artificial intelligence (AI)-enabled camera systems to enhance patient safety, addressing risks like falls and bedsores, but leaders are also acknowledging challenges related to patient consent, compliance, and privacy. The San Diego Union-Tribune reported the news January 5. According to the article,…
3D modeling advances cardiovascular surgery at Boston Children's Hospital

Editor's Note Computational fluid dynamics (CFD), a technique historically used in engineering, is transforming pediatric cardiac surgery at Boston Children's Hospital, Healthcare IT News reported January 2. This approach aims to enhance surgical precision and reduce the need for repeat operations in children with congenital heart defects. Traditionally, pediatric heart…
Report: Healthcare ransomware compromises millions of patient records, costs billions in downtime

Editor's Note Between 2018 and 2024, ransomware attacks on US healthcare organizations compromised nearly 89 million patient records and resulted in downtime costing an estimated total of $21.9 billion, according to a December 18 report from Comparitech. The report tallies 654 total ransomware incidents during this period targeting hospitals, clinics,…
Hospitals continue to grapple with IV fluid shortage

Editor's Note US hospitals continue to face a shortage of intravenous (IV) fluids due to Hurricane-related damage to the Baxter manufacturing facility that was responsible for 60% of the nation’s supply, MedPage Today reported December 31. Citing a report from Baxter, the article notes that the manufacturer has restored eight…
Proposed tariffs, stricter immigration policies threaten healthcare workforce, supply chain
Editor's Note Immigration and trade policies proposed by President-elect Donald Trump could exacerbate challenges with healthcare staff and medical supply chains, according to recent reporting from Modern Healthcare and The Hill. As detailed by Modern Healthcare, stricter immigration policies could impede efforts to recruit skilled international workers, exacerbating gaps in…
Visual management supports perioperative Lean efforts

Takeaways • Visual management (VM) tools support Lean efforts, leading to improved quality and efficiency. • VM boards provide an overview of processes, facilitate problem solving, and promote staff-manager collaboration. • Other VM tools such as color coding and customized instrument trays can help reduce errors and save money. Lean…
Why active implants demand proactive management
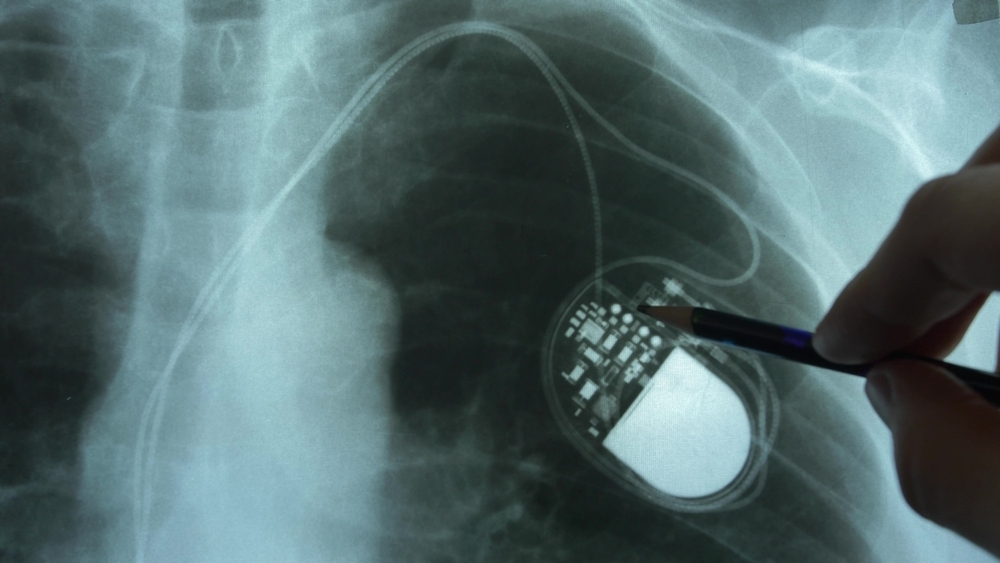
What happens when a surgeon uses the monopolar instrument set on 30-W coagulation mode to create an upper midline incision in a patient with a pacemaker? Pacemaker function is interrupted, causing a heart block that results in hemodynamic instability—or at least, this is what could happen without taking the necessary…
Orthopedic research showcases stem cells’ surgical promise
Stem cell therapy is poised to revolutionize regenerative medicine. As these therapies advance, they may alter or even replace certain invasive surgical procedures. Already undergoing advanced clinical trials, orthopedic applications are likely candidates for attracting the earliest adopters. Results so far indicate significant potential for providing alternatives to implants, grafts,…
Implications of 2024 surgical care trends for the year ahead
Perioperative leaders are entering a time of uncertainty after 2024 proved to be transformative for inpatient and outpatient surgical care. Last year saw important updates from The Joint Commission and the Centers for Medicare & Medicaid Services (CMS) that emphasized improvements alongside ongoing challenges in safety, workforce shortages, reimbursement, and…

 Free Daily News
Free Daily News