 Supply Chain/Technology
Supply Chain/Technology
Offsite sterilization fuels onsite efficiency for lean ASCs

For many in the healthcare industry, imagining surgery without onsite sterile processing seems unthinkable. Then again, performing total joints in an ambulatory surgery center (ASC) was unthinkable 10 years ago. ASC sterile processing departments (SPDs) are generally not designed to handle the high volumes of instrument trays, vendor trays, and…
Water quality: 5 Ws and an H for sterile processing pros

Asking who, what, why, when, where, and how—otherwise known as the “5 Ws and an H”— is a time-tested way for writers and researchers to ensure comprehensive coverage of any topic. Here, we apply this framework from the perspective of sterile processing department (SPD) professionals seeking to start a water…
Staffing solutions depend on collaborative, innovative culture

There is no shortage of advice, opinions, and proposed solutions when it comes to staff shortages, but the issue continues to plague healthcare systems nonetheless. For a couple of years now, speakers at the OR Business Management Conference and OR Manager Conference have been asking attendees, “Who still struggles to…
Pilot project takeoff proves promise of AI staff optimization
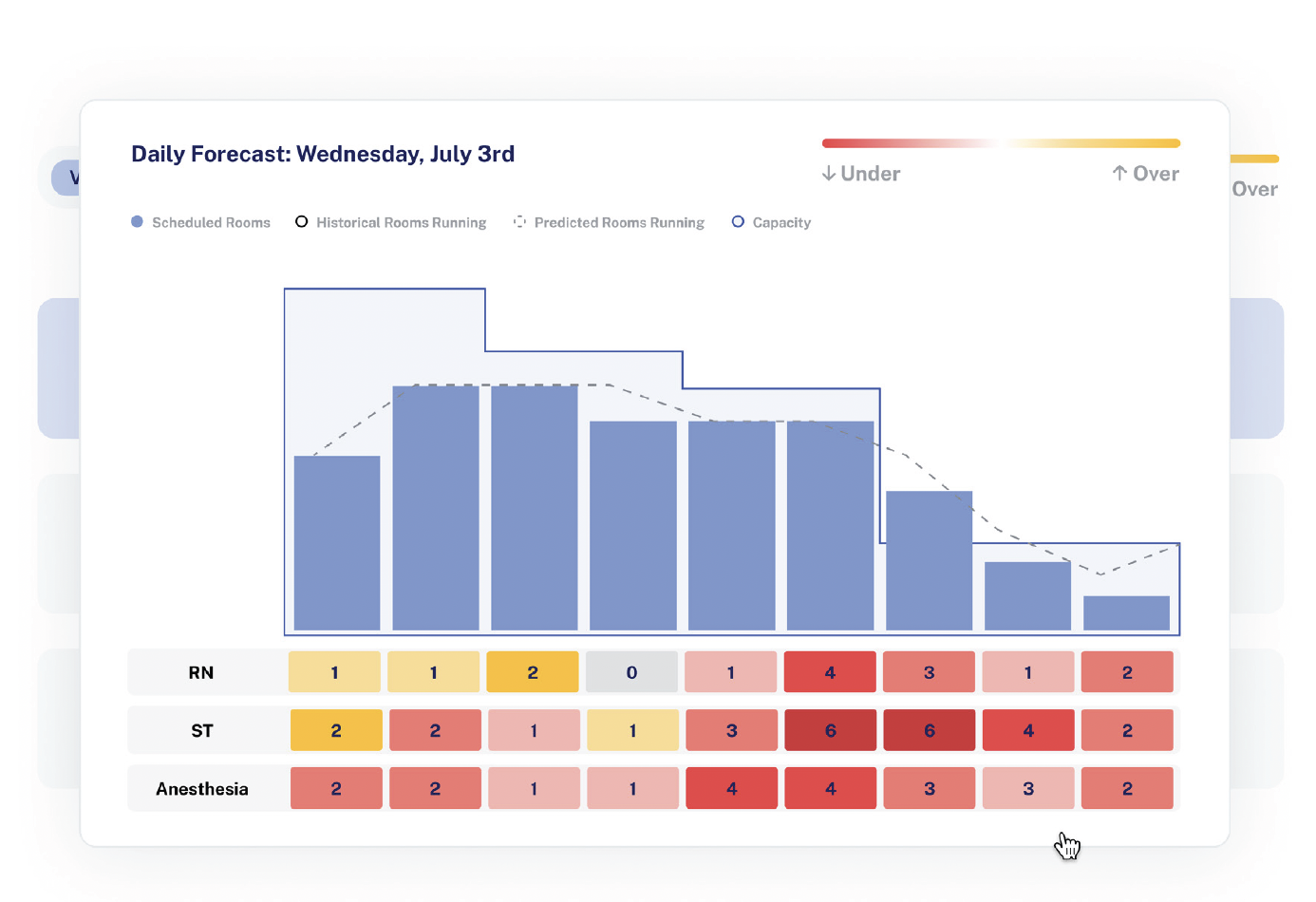
Perioperative leaders face mounting pressures to optimize resources, reduce costs, and improve patient outcomes. However, one challenge stands out among the rest: OR staffing shortages. According to a November/December survey conducted by LeanTaaS in collaboration with OR Manager, staff recruitment and retention is a top priority for OR leaders this…
FDA approves single-dose propofol amid drug shortage

Editor's Note The Food and Drug Administration (FDA) approved Amneal Pharmaceuticals' Abbreviated New Drug Application for single-dose vials of propofol injectable emulsion, Anesthesiology News August 23 reports. The newly approved formulations include 200 mg/20 mL, 500 mg/50 mL, and 1,000 mg/100 mL vials. Propofol is widely used for anesthesia and…
Some FDA-recalled medical devices remain in use while manufacturers implement corrections

Editor's Note A 2016 recall issued for the Abbott MitraClip cardiac device highlighted potential safety concerns, but instead of removing the product from the market, Abbott and the Food and Drug Administration (FDA) allowed continued use with revised instructions and additional training for doctors. This approach reflects a broader trend…
International study highlights OR waste management strategies, barriers

Editor's Note Effective waste segregation and adopting a circular economy approach can significantly reduce environmental impact of incinerating hospital waste incorrectly classified as hazardous, according to a narrative review published August 19 in the Medical Journal of Australia. However, surgeons' concerns about patient safety and insufficient systemic policies can hinder…
Handheld, powered surgical tools combine advantages of traditional laparoscopy, robotics

Editor's Note Combining dexterity and cost-effectiveness, handheld robotic devices offer potential to bridge the gap between traditional laparoscopy and more expensive robotic platforms, researchers concluded August 8 in the journal Surgery. The mini-review of clinical trials covered clinical applications of three handheld robotic devices: the HandX powered laparoscopic instrument from…
How GLP-1 drugs impact health systems expansion, investment plans

Editor's Note A shuttered bariatric surgery center in Oklahoma last month is just one example of how the rise of Glucagon-like peptide-1 (GLP-1) agonists are reshaping health systems’ investments, Axios reported August 13. Rather than “massive hospital towers with cardiology clinics, dialysis beds and joint replacement centers,” the focus is…
FDA announces Class 1 recall for chest compression devices

Editor's Note The US Food and Drug Administration (FDA) has designated Defibtech, LLC’s recall of RMU-2000 ARM XR Chest Compression Devices as Class 1, the most severe category indicating serious risk of injury or death. A motor issue could stop compressions in adults whose hearts suddenly stop, according to the…

 Free Daily News
Free Daily News