 Supply Chain/Technology
Supply Chain/Technology
FDA: Class I recall of Draeger Medical’s Seattle PAP Plus, breathing circuit/anesthesia kits
Editor's Note The Food and Drug Administration (FDA), on May 24, identified the recall by Draeger Medical of the Seattle PAP Plus as well as VentStar and other breathing circuit/anesthesia kits as Class I, the most serious. The recall was initiated after finding that glued connections may loosen before or…
New EHR generative AI by Microsoft, Epic currently being piloted
Editor's Note On April 17, electronic health records (EHR) vendor Epic and Microsoft announced a partnership to train Azure OpenAI on a large collection of information so it can “asynchronously draft responses to patient messages for providers,” Becker’s Health IT May 25 reports. According to Becker's, there are four major…
FDA: Class I recall of certain ICU Medical infusion system batteries
Editor's Note The Food and Drug Administration (FDA), on May 22, identified the recall by ICU Medical of replacement batteries for its Plum 360, Plum A+, and Plum A+3 infusions systems as Class I, the most serious. The recall was initiated because a manufacturing defect substantially diminished how long the…
AAMI releases updated protective barriers standard

The Association for the Advancement of Medical Instrumentation (AAMI) has revised the ANSI/AAMI PB70 Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities. This revised standard provides healthcare professionals with a better understanding of the barrier protection properties of available protective apparel. The…
SSI prevention in ASCs
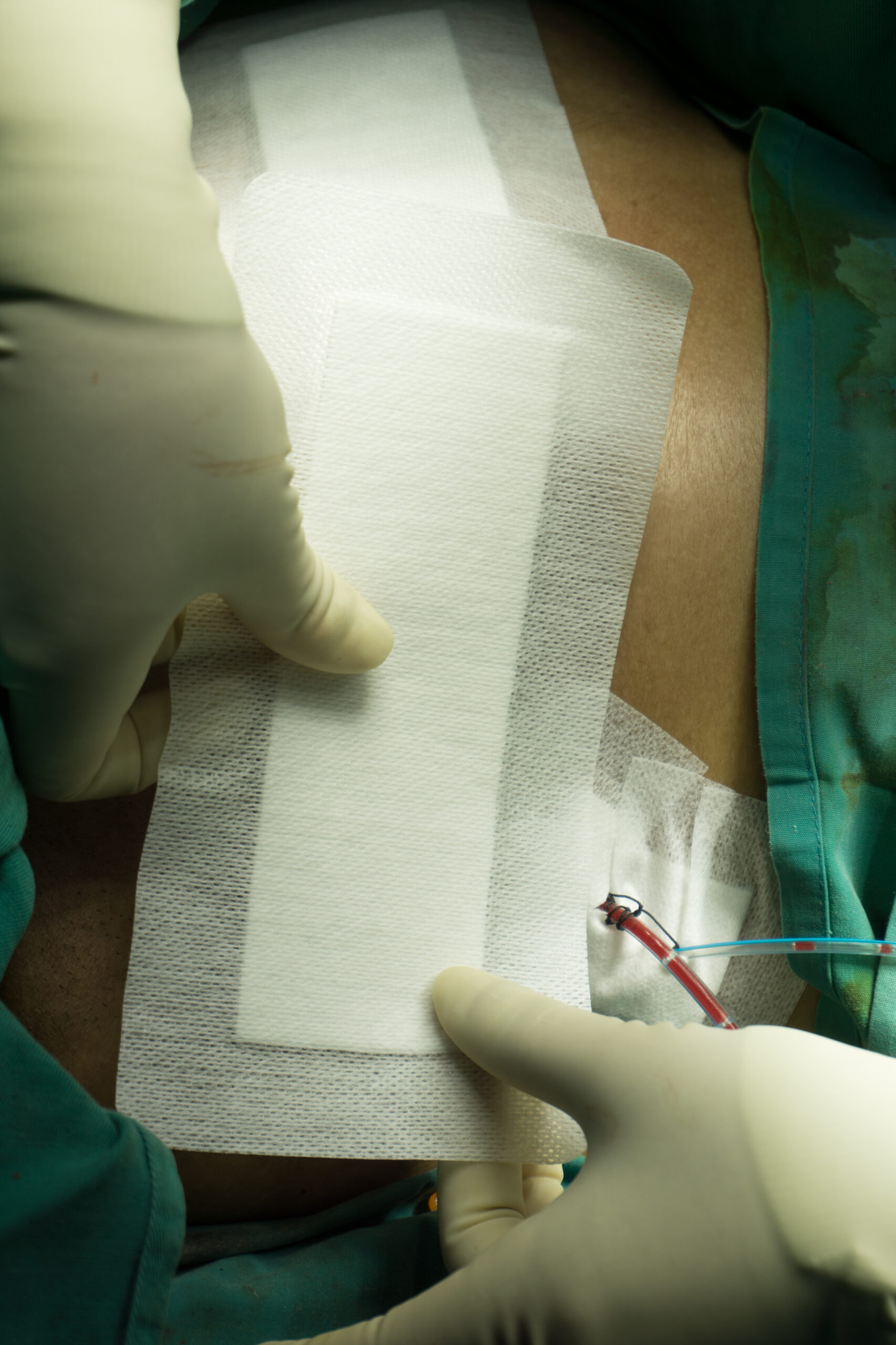
Surgical site infection (SSI) surveillance has rapidly grown in the ambulatory setting over the last decade, with the expansion of the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network’s (NHSN) Outpatient Procedure Component (OPC). OPC-SSI is designed to track and monitor SSIs in ambulatory surgery centers (ASCs)…
ECRI announces 2023 Alerts Impact Award winners
Editor's Note ECRI, on May 18, announced the winners of its annual 2023 Alerts Impact Award. The Award is given to members of ECRI’s Alerts Workflow system for excellence in recall management. The system’s software is used by supply chain, clinical engineering, IT, pharmacy, lab, clinical departments, and ancillary-care points…
Heightened focus on cybersecurity amid ransomware attacks, data breaches
Editor's Note Cybersecurity remains a top concern and potential hazard for patient data. In February, Lehigh Valley Health Network in Allentown, Pennsylvania, was attacked by a ransomware gang BlackCat, which has ties to Russia, Becker’s Health IT reports. The health system has since notified 627 individuals and will continue to…
Problems persist with HHS emergency-preparedness goals
Editor's Note According to a May 14 Government Accountability Office (GAO) report, the US Department of Health and Human Services (HHS) is continuing with the persistent, systemic problems that impaired its ability to respond to the COVID-19 pandemic as well as other public health crises, such as H1N1 influenza, Zika,…
XBB.1.16 grows to 14% of US COVID-19 cases
Editor's Note Estimates from the Centers for Disease Control and Prevention find the emerging COVID-19 Omicron subvariant XBB.1.16, dubbed Arcturus, is responsible for more than 14% of COVID-19 cases in the US over the past 2 weeks, up from nearly 7% in the prior 2 weeks, according to the May…
HHS issues alert on cyber attacks against Veeam software
Editor's Note The Department of Health and Human Services’ (HHS) Health Sector Cybersecurity Coordination Centers on May 10 issued an alert saying that cyber attacks against Veeam Backup & Replication software are on the rise, the American Hospital Association reports. What makes the threat significant is that this software is…

 Free Daily News
Free Daily News