 Supply Chain/Technology
Supply Chain/Technology
FDA issues warning letters to endoscope manufacturer
Editor's Note The Food and Drug Administration (FDA) on January 10 announced that it had recently issued two warning letters to Olympus Medical Systems Corporation and one of its subsidiaries, Aizu Olympus Co, Ltd, after facility inspections. The letters addressed violations related to medical device reporting (MDR) requirements and quality…
Allina Health and Qventus using AI tool for OR scheduling
Editor's Note Allina Health and software company Qventus partnered earlier this year to implement an artificial intelligence-based tool to help automate OR scheduling, according to a January 10 PR Newswire release, reports January 11 Becker’s Hospital Review. The tool has capabilities to integrate into electronic health record systems and uses…
FDA: Class I recall of Arrow central venous access and catheter kits
Editor's Note The Food and Drug Administration (FDA) on December 16 identified the recall by Teleflex and Arrow International, LLC, of its Arrow MAC Two-Lumen Central Venous Access Kits and Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits as Class I, the most serious. The recall…
Utility of lighted magnification, borescopes to inspect flexible endoscopes
Editor's Note This study led by epidemiologist Cori L. Ofstead, MSPH, and associates, St Paul, Minnesota, found visible damage and residue or debris in 100% of 25 processed flexible endoscopes, using a new visual inspection program that included magnification and borescopes. Fully processed endoscopes were examined twice during a 2-month…
FDA: Class I recall of Arrow AutoCAT2, AC3 IABPs
Editor's Note The Food and Drug Administration (FDA), on December 20, identified the recall by Arrow International, LLC, subsidiary of Teleflex, Inc, of its Arrow AutoCAT 2 and AC3 intra-aortic balloon pumps (IABPs) as Class I, the most serious. IABPs are used in patients having cardiac and non-cardiac surgery, and…
Deloitte surveys 2023 outlook for healthcare systems, plans
Editor's Note Hospital leaders are preparing for what is expected to be a turbulent 2023, according to results of a December 13 survey from the Deloitte Center for Health Solutions, reports December 19 Healthcare Purchasing News. The majority of health system leaders said that staffing challenges (85%) and inflation (76%)…
State of natural language processing in surgery: Part 2

Takeaways Natural language processing (NLP) may be helpful for predicting surgical outcomes and can facilitate more robust research. NLP comes with challenges that include potential for bias, costs, technology and terminology barriers, and the need to adapt to new ways of thinking. Before purchasing products that use NLP, OR leaders…
New AMDR report strengthens case for single-use medical device reprocessing

Recent analysis provided by the Association of Medical Device Reprocessors (AMDR) indicates that US hospitals could save up to $2.28 billion a year by maximizing the use of reprocessed single-use medical devices. According to the report, in 2020, US hospitals saved $372 million just by reprocessing single-use medical devices, as…
Key lessons on supply management at OR Manager Conference

Two major challenges that perioperative leaders are facing today are labor and supply costs. These two areas also happen to be the largest and second largest expense categories at most hospitals, making the challenges even more pronounced. What actions can leaders put in place to ensure that clinical staff are…
Tackling internal objections to single-use device reprocessing
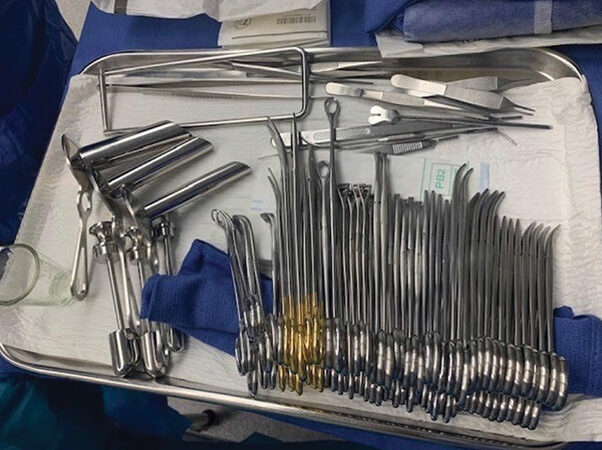
Many healthcare professionals are aware of single-use device reprocessing and its benefits. According to the Association of Medical Device Reprocessors, the savings generated by such programs can strengthen a facility’s financial sustainability and help provide a path for more responsible environmental stewardship. Sometimes, ambulatory surgery centers (ASCs) may experience situations…

 Free Daily News
Free Daily News