Editor's Note Perioperative nurses engaging in patient and community education to explain coronary artery bypass grafting (CABG) surgery, reinforcing recommended discharge guidelines, and serving as expert resources are key to positive outcomes and reducing readmissions, finds this review article. In 2016, the Centers for Medicare & Medicaid Services reduced payments…
Editor's Note The Food and Drug Administration (FDA) on October 31 issued a Safety Alert saying that interim study results continue to show an increased rate of major adverse cardiac events and Bioresorbable Vascular Scaffold (BVS) thrombosis in patients receiving the Absorb GT1 BVS by Abbott Vascular (Abbott Park, Illinois),…
Editor's Note The Food and Drug Administration (FDA) on October 19 issued an updated Safety Alert for implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators by St Jude Medical. Because batteries for the devices may fail earlier than expected, St Jude has made available a new battery performance management tool,…

Editor's Note Wide variation was found in 90-day coronary artery bypass grafting (CABG) episode payments for Medicare and private payer patients in this study. The differences were driven by increased use of evaluation and management services, higher utilization of inpatient rehabilitation, and patients with multiple readmissions. In the analysis of…

Editor's Note In this multicenter study, on-pump coronary artery bypass grafting (CABG) led to significantly higher rates of 5-year survival and event-free survival than off-pump CABG. From 2002 to 2007, a total of 2,203 patients at 18 medical centers were randomly assigned to undergo either on-pump (1,099 patients) or off-…
Editor's Note The Centers for Medicare & Medicaid Services (CMS) on August 15 announced a proposed rule that would cancel two bundled-payment models and reduce the number of providers required to participate in a third. The proposed rule would cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment…
Editor's Note A research report by Marketsandmarkets predicts the global hybrid OR market will reach nearly $1.2 billion by 2022, at a 12.5% annualized growth rate, according to the August 14 HealthCareBusiness daily news. The specialty with the highest growth rate will be thoracic surgery. The biggest driver of hybrid…
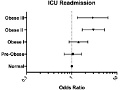
Editor's Note After cardiac surgery, obese patients required significantly more ICU resources and longer recovery times, resulting in more expensive, labor-intensive care, this study finds. Of 5,365 patients included in the analysis, 1,948 were classified as obese. Patients with greater obesity were: four times more likely to have longer time…

Editor's Note The Food and Drug Administration (FDA) on August 4 classified the recall by Datascope Corp/Maquet (Mahwah, New Jersey) of its intra-aortic balloon pump as Class I, the most serious. The recall was issued because of the risk of a valve failure, which prevents the balloon from inflating and…

Editor's Note The Joint Commission on July 19 announced that the University of Kansas Health System, Kansas City, is the first hospital in the nation to achieve its Comprehensive Cardiac Center (CCC) Certification. To achieve certification, hospitals must demonstrate compliance with consensus-based standards, evidence-based clinical practice guidelines for cardiac care,…