
Editor's Note Hospitals that succeed in surveys are those that “hardwire safety so you’re not ramping up and down,” said John R. Rosing, MHA, FACHE, executive vice president and principal of Patton Healthcare Consulting. Speaking at the OR Manager Conference, Rosing reminded perioperative leaders, “If we’re really about providing quality…

The centralization of medical device processing to one facility is becoming more prevalent. Centralizing sterile processing activities reduces expenses while concentrating expertise. However, this also introduces new concerns. When sterile processing is located within the same building where instrumentation is used, transport occurs over smooth floors in a controlled environment…
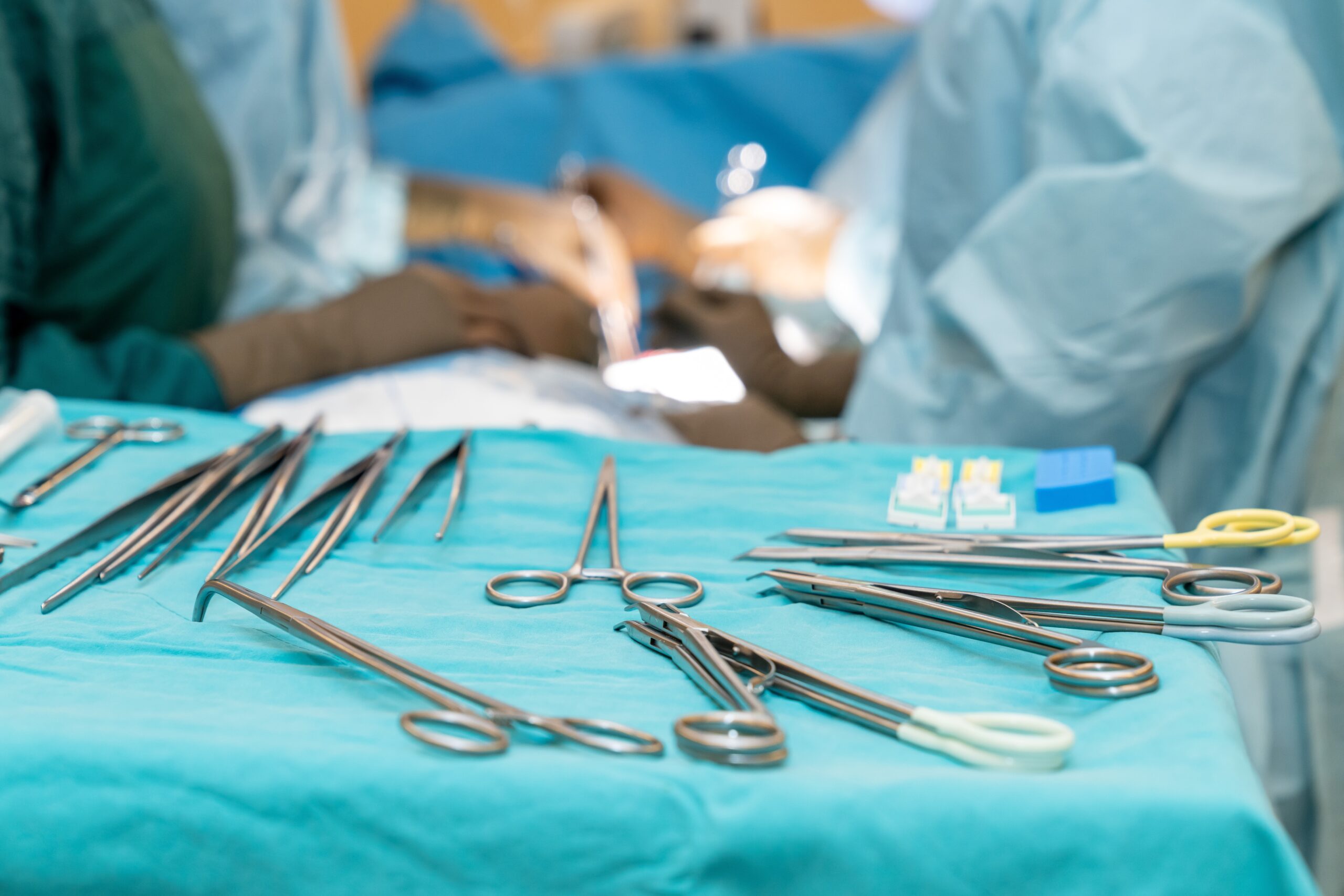
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has released the updated AAMI TIR17:2024; Compatibility of Materials Subject to Sterilization, its first revision since 2017. This guidance provides essential information for medical device manufacturers, designers, and sterilization professionals on how sterilization methods impact materials and packaging. Updates…

Editor's Note New guidance from The Association for the Advancement of Medical Instrumentation (AAMI) addresses regulatory and safety concerns for hospitals using ethylene oxide (EO) for medical device sterilization—a pressing concern due to EO’s effectiveness but associated health risks. ANSI/AAMI ST58:2024, an update on chemical sterilization and high-level disinfection practices…

Asking who, what, why, when, where, and how—otherwise known as the “5 Ws and an H”— is a time-tested way for writers and researchers to ensure comprehensive coverage of any topic. Here, we apply this framework from the perspective of sterile processing department (SPD) professionals seeking to start a water…

Editor's Note In a new guidance document for manufacturers of pharmaceuticals and biopharmaceuticals, The Association for the Advancement of Medical Instrumentation (AAMI) has released a new guidance document updating best practices for radiation sterilization validation and routine control of single-use systems. The document, AAMI CR513:2024; Guidance on radiation sterilization validation and…

Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has updated its standard on ethylene oxide (EO) sterilizers for healthcare facilities, according to a June 12 press release. The first update in two decades, the fourth edition ANSI/AAMI ST24:2024 covers labeling, safety, performance, and testing requirements for general-purpose…

Editor's Note A new guidance document covering the entire process for the selection, labeling, and sterile processing of dilators and ultrasound probes is available from The Association for the Advancement of Medical Instrumentation (AAMI). Released April 17, AAMI TIR99:2024; Processing Of Dilators, Transesophageal And Ultrasound Probes In Health Care Facilities…