
Editor's Note Prostate cancer surgeries for low-risk patients have plummeted since 2010, signaling major progress in reducing overtreatment, according to an April 29 announcement from the University of Michigan. University researchers reportedly found that the proportion of men undergoing prostatectomy for Grade Group 1 prostate cancer—the lowest-risk category—dropped more than…

Editor's Note Recent reporting from Fierce Healthcare highlights two notable technology advances that promise to impact surgical patients and caregivers: an AI-driven blood pressure monitoring system and a remote-controlled endovascular robotics platform. The first development, from BD, is designed to help clinicians anticipate and manage dangerous blood pressure drops during…

Editor's Note Pulse oximeters may overestimate blood oxygen levels in critically ill patients with darker skin tones, according to a March 30 article in HCP Live. The article focuses on the EquiOx study, conducted at the Zuckerberg San Francisco General Hospital between 2022 and 2024. Presented at the American College…

Editor's Note The Smidt Heart Institute at Cedars-Sinai has introduced a pioneering device aimed at treating patients with difficult-to-manage hypertension, making it one of four institutions in the US and the first on the West Coast to make use of this technology, a March 8 press release published by Cedars…
Editor's Note Researchers at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and the University Hospital Dresden (UKD) have developed a portable, droplet-based millifluidic device that can monitor patients for postoperative pancreatic fistula in the critical first days after surgery. The same technology might also be expanded to analyze other body fluids and diseases.…
Editor's Note The Essenz™ In-Line Blood Monitor (ILBM) has received U.S. FDA 510(k) clearance and CE mark, as announced by manufacturer LivaNova on August 30. The new ILBM provides “accurate and continuous measurement of essential blood parameters for perfusionists throughout cardiopulmonary bypass procedures,” and integrates with the company’s Essenz Perfusion…
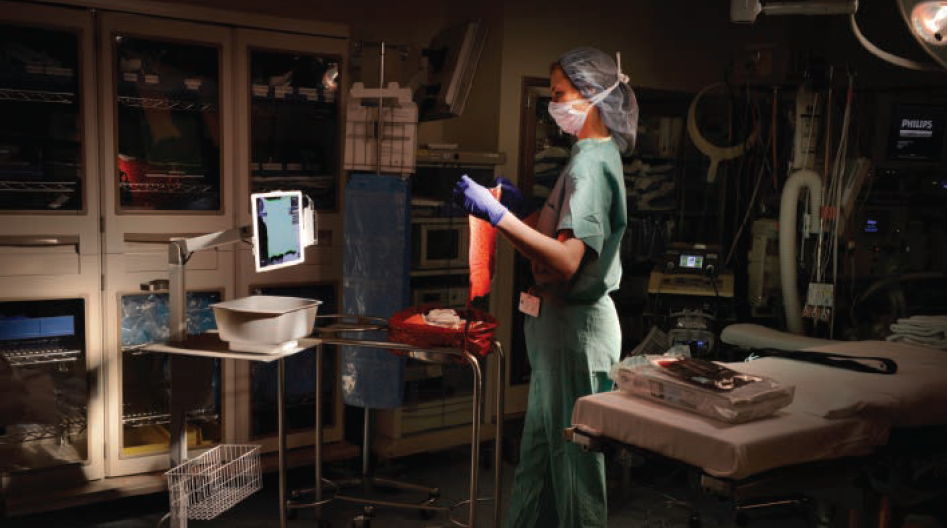
Blood loss during labor and delivery (L&D) and surgical procedures can lead to serious complications that might be prevented with early detection; however, detection can be challenging. For example, clinicians have traditionally estimated blood loss visually—a subjective and often inaccurate process. Humans’ eyes simply aren’t good at making precise measurements,…