
Editor's Note A Phase III clinical trial comparing transcatheter aortic valve replacement (TAVR) with traditional open-heart surgery found no significant differences in key health outcomes 7 years after treatment, Cedars-Sinai October 27 reports. The international PARTNER 3 trial, led by Raj Makkar, MD, and published in The New England Journal…

Editor's Note Robotic-assisted cardiac surgery is reshaping the field, enabling safer, less invasive procedures once deemed too complex for minimal-access techniques, the American College of Surgeons October 1 reports. Advances in high-definition visualization, wristed instruments, and surgical control are allowing cardiothoracic teams to perform intricate repairs through incisions often smaller…

Editor's Note Private equity firms are accelerating their push into outpatient cardiology, lured by reimbursement trends and a fragmented market but shadowed by concerns about patient outcomes and financial motives, Modern Healthcare October 16 reports. Investor activity in cardiology has surged alongside the Centers for Medicare & Medicaid Services (CMS)…

Editor's Note Children’s Health in Dallas leads the nation in pediatric orthopedic surgery, after the US News & World Report 2025–2026 Best Children’s Hospitals rankings showcased familiar national leaders across a range of pediatric specialties, D Magazine and Fierce Healthcare October 7 report. The latest results highlight continued excellence in…

Editor's Note The Food and Drug Administration (FDA) on October 10 classified a cybersecurity correction involving Abiomed’s Automated Impella Controller as a Class I recall, the most serious type, according to the FDA Medical Device Recalls and Early Alerts database. While devices are not being removed from clinical settings, the…

Editor's Note The Food and Drug Administration (FDA) has issued multiple high-risk medical device recalls in recent weeks, mid-September FDA announcements report. On August 21, Medline alerted customers that some of its convenience kits contain recalled Medtronic DLP Left Heart Vent Catheters. These catheters, used in cardiopulmonary bypass, may fail…

Editor's Note Minimally invasive surgery can extend beyond valve replacement to complex aortic procedures without sacrificing safety or long-term outcomes. According to an August 21 news update from the University of Miami Miller School of Medicine, a new study of 796 patients found adding ascending aortic or hemiarch replacement to…

Editor's Note A freezing technique applied during heart surgery is reducing pain, shortening recovery times, and minimizing the need for narcotics, News 9/CBS News August 13 reports. The procedure, called cryo nerve ablation, involves freezing nerves around the ribs to block pain signals for about 60 days. The nerves eventually…

Editor's Note Patients from socioeconomically deprived areas are more likely to have reduced cardiorespiratory fitness before surgery, potentially contributing to poorer surgical outcomes, MedicalXpress August 12 reports from a study published by PLOS One. The research, led by PhD student Donna Shrestha of Lancaster University Medical School, analyzed preoperative fitness…
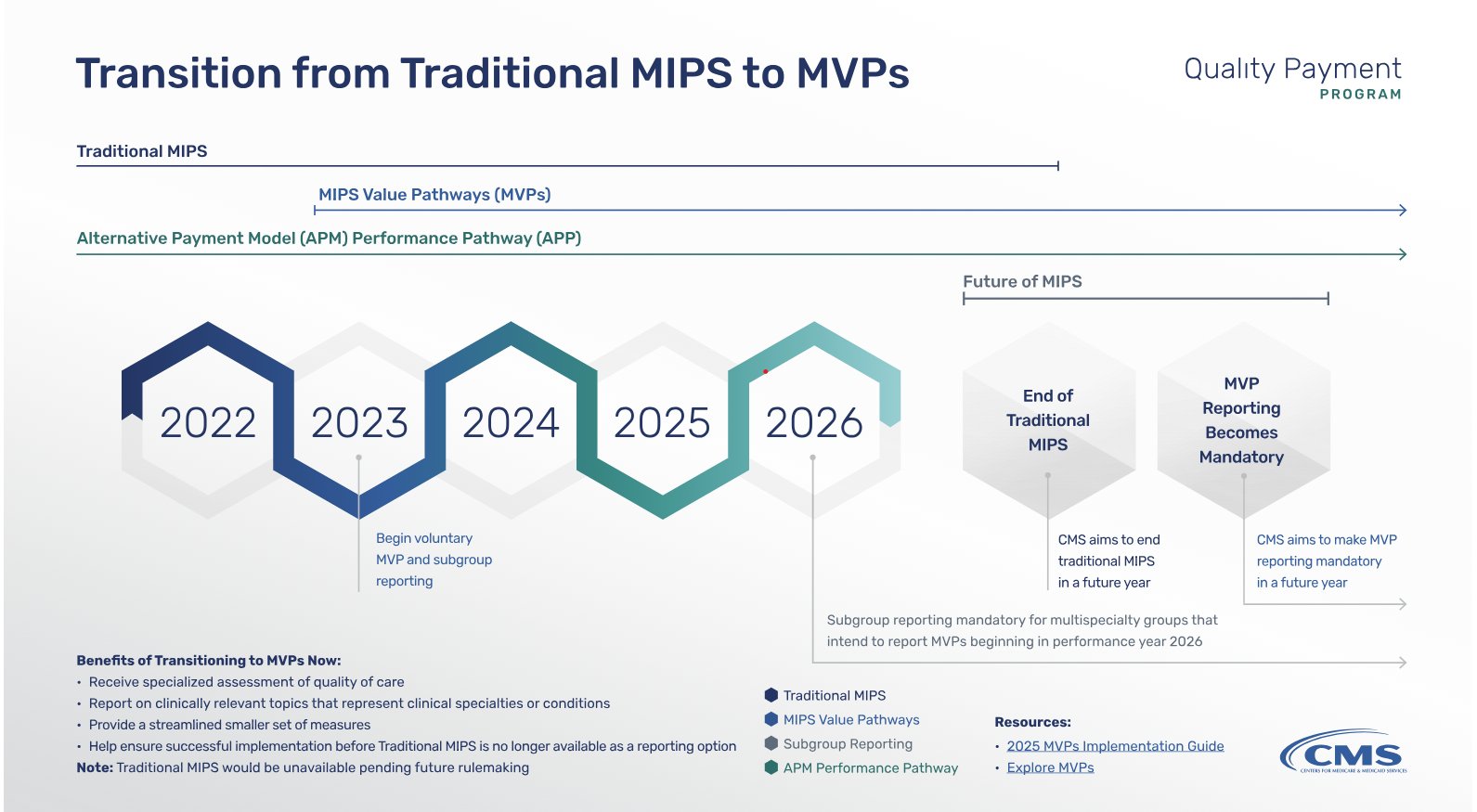
In July 2025, the Centers for Medicare & Medicaid Services (CMS) proposed sweeping changes to the Medicare Physician Fee Schedule for calendar year 2026. Among the most impactful updates is the launch of the Ambulatory Specialty Model (ASM)—a mandatory value-based payment program focused on heart failure and low back pain.…