For decades, ambulatory surgery centers (ASCs) have shown their ability to deliver high-quality surgical care at substantially lower cost than hospital outpatient departments (HOPDs). ASCs achieve these savings through leaner operations, streamlined staffing models, and specialty-focused efficiencies, not by compromising safety or outcomes. Studies consistently highlight procedures performed in ASCs…

Editor's Note Aortic root cannulas from Medtronic are the subject of the latest US Food and Drug Administration (FDA) class 1 recall, the most serious category reserved for risk of injury or death. Affected products include the DLP Aortic Root Cannula, MiAR Cannula, and DLP Aortic Root Cannula with Vent…

Editor's Note Miniaturized pacemakers implanted in neonates and infants have demonstrated reliable performance for up to two years, with no unexpected device failures, according to a March 11 article in Healio. The research, published in Circulation: Arrhythmia and Electrophysiology, suggests that these modified pacemakers could offer a viable alternative for…

Editor's Note An Australian man lived for 100 days with an artificial titanium heart, the longest duration recorded for a patient using the device, according to a March 12 report from CNN. The breakthrough marks a significant step toward using total artificial hearts as a long-term solution for patients with…

Editor’s Note The US Food and Drug Administration (FDA) has designated Boston Scientific Corporation’s recall of Accolade Pacemaker devices a Class 1, the most severe category indicating serious risk of injury or death. According to the agency’s February 21 announcement, the recall was motivated by a manufacturing issue that could…

Editor's Note Patients aged 50 to 70 undergoing heart valve replacement may benefit from mechanical valves over biological ones, according to a new study from the University of Bristol. Healthcare-in-Europe.com reported the news February 13. As detailed in the article, short-term outcomes between the two options were similar. However, mechanical…

Editor's Note Coronary artery bypass graft (CABG) surgery and transcatheter aortic valve replacement (TAVR) generate the highest overall financial returns for US hospitals, despite not being the most profitable per procedure, according to a February 13 report in Cardiovascular Business. The article covers an analysis of Medicare data, published in…
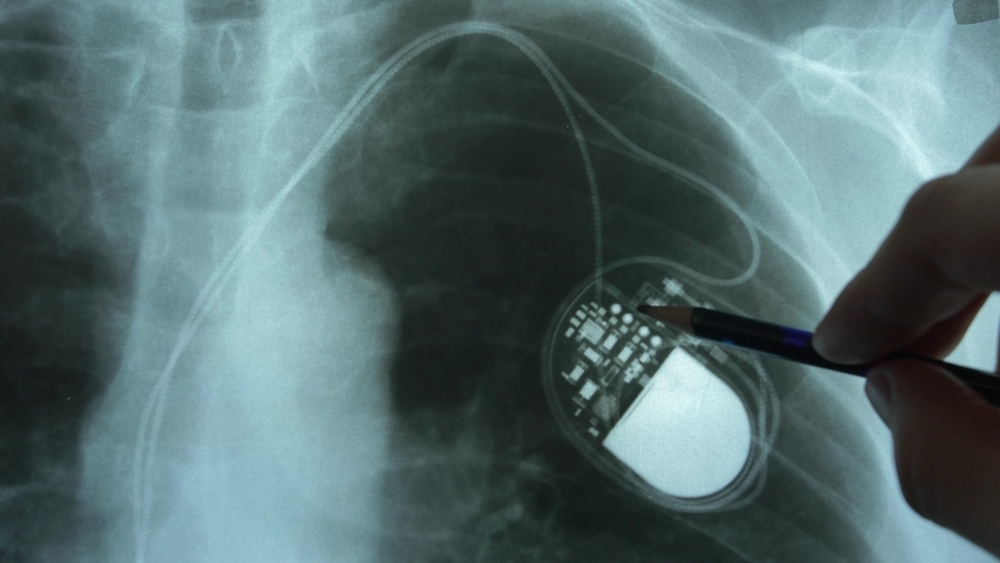
What happens when a surgeon uses the monopolar instrument set on 30-W coagulation mode to create an upper midline incision in a patient with a pacemaker? Pacemaker function is interrupted, causing a heart block that results in hemodynamic instability—or at least, this is what could happen without taking the necessary…

Editor's Note The lack of available hearts for transplantation combined with the recent recall of Abbott’s HeartMate 3 left ventricular assist device (LVAD) “makes the current therapy landscape for heart failure much more dire,” according to a May 20 report in Medical Device Network. The recall of the device, which…

Editor's Note More than 45 years after surgery, Mary Ann Kozlowski’s mechanical heart valves are still pumping—and Guinness is reviewing an application to list her as the new world record-holder for longevity of a double-valve replacement. That’s according to a May 15 report in the Erie-Times News, which detailed how…