
Editor's Note The Food and Drug Administration (FDA) has issued a series of early alerts this month regarding high-risk recalls from several leading medical device makers, citing patient safety risks ranging from pump failures to vascular complications. These alerts highlight issues with products from Johnson & Johnson’s (J&J’s) Abiomed unit,…

Editor's Note Researchers have developed a fully dissolvable, needle-injectable pacemaker that regulates heart rhythms without requiring surgical removal. As detailed in an April 2 article in Scientific American, the miniature device—just millimeters in size—can deliver electrical stimulation for days to weeks before safely breaking down in the body, potentially reducing…

Editor's Note The US Food and Drug Administration (FDA) designated Getinge’s recall of Vaporizer Sevoflurane Maquet Filling and Sevoflurane Quick-Fil and Philips’ recall of Tack Endovascular Systems as Class 1s, the most severe category indicating serious risk of injury or death. The Getinge recall is an expansion of a 2024…

Editor's Note Coronary artery bypass graft (CABG) surgery and transcatheter aortic valve replacement (TAVR) generate the highest overall financial returns for US hospitals, despite not being the most profitable per procedure, according to a February 13 report in Cardiovascular Business. The article covers an analysis of Medicare data, published in…

Editor’s Note At the Society of Thoracic Surgeons (STS) annual meeting, experts urged cardiac surgeons to become more engaged in tricuspid valve treatment before transcatheter options gain too much traction according to a January 26 report in MedPage Today. With new transcatheter devices gaining FDA approval and the Centers for…
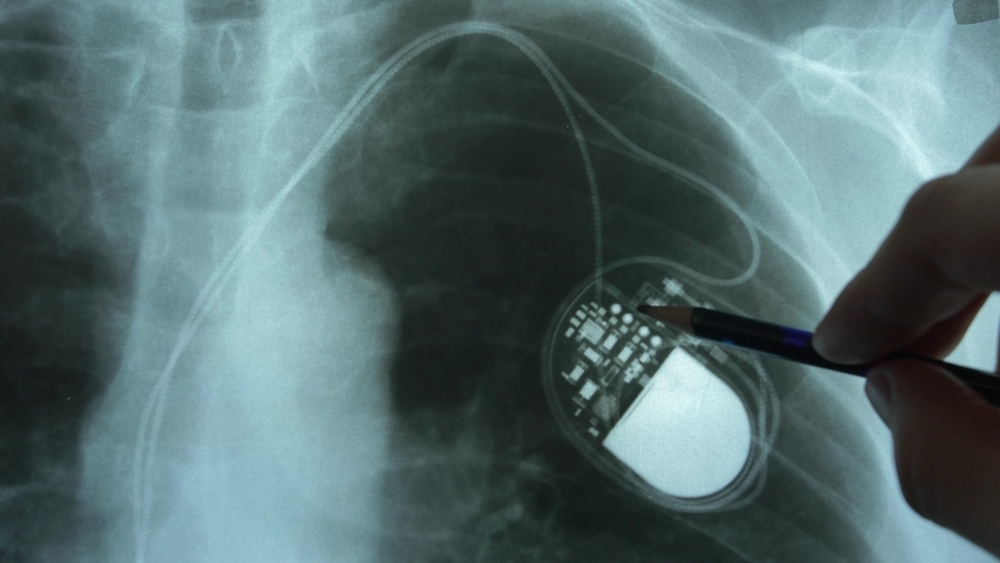
What happens when a surgeon uses the monopolar instrument set on 30-W coagulation mode to create an upper midline incision in a patient with a pacemaker? Pacemaker function is interrupted, causing a heart block that results in hemodynamic instability—or at least, this is what could happen without taking the necessary…

Editor's Note Cardiothoracic surgeons at Emory University Hospital have conducted the first US implantation of the BrioVAD System, a new ventricular assist device (VAD) from BrioHealth Solutions, Cardiovascular Business News reported November 25. According to the article, the BrioVAD System features a magnetically suspended, hemocompatible pump designed to minimize adverse…

Editor's Note A September 2024 study, published in the Journal of the American College of Cardiology, found that subclinical atherosclerosis progression in asymptomatic individuals is strongly linked to increased risk of death from any cause, CathLab Digest September 30 reports. The study, led by Mount Sinai Fuster Heart Hospital researchers,…

Editor's Note Using a device they call a “space hairdryer,” researchers in Austria applied gentle shockwaves to regenerate heart tissue after coronary artery bypass graft surgery (CABG) in a study with potential implications for millions of patients, BBC News reported June 20. Researchers are now seeking larger trials, European regulatory…

Editor's Note The US Food & Drug Administration (FDA) has announced Class 1 recalls—the most severe category, indicating risk of serious injury or death—for three products: MEGA SOFT Pediatric Patient Return Electrodes from Megadyne, Vaporizer Sevoflurane Maquet Filling from Getinge; and Arrow FiberOptix Intra-Aortic Balloon Catheter Kit and Arrow UltraFlex…