
Editor's Note New and revised workplace violence prevention requirements take effect for The Joint Commission-accredited, office-based surgery practices July 1, The Joint Commission announced. According to the January 16 announcement, which also announced new and revised standards for accredited assisted living communities and nursing care centers, the updates aim to…

Editor's Note The US Food and Drug Administration (FDA) has issued new draft guidance January 6 providing recommendations improving the use of pulse oximeters and supporting the safety of AI-enabled medical devices, among other topics. Released January 6, draft guidance supporting the safety and effectiveness of AI-enabled devices would, if…

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has released the updated AAMI TIR17:2024; Compatibility of Materials Subject to Sterilization, its first revision since 2017. This guidance provides essential information for medical device manufacturers, designers, and sterilization professionals on how sterilization methods impact materials and packaging. Updates…

Editor's Note In a move that could hinder provider-led joint ventures and increase regulatory uncertainty, the Federal Trade Commission (FTC) and the Justice Department have withdrawn antitrust guidance issued in 2000. Modern Healthcare reported the news on December 16. Passed in a 3-2 vote, the decision eliminates previously relied-upon safe…

Editor's Note Efforts to reduce the risk of postoperative delirium in older patients should focus on preoperative evaluation, anesthesia choices, and medication management, according to the American Society of Anesthesiologists (ASA). Designed specifically for treating adults aged 65 and older undergoing inpatient surgery, these new, evidence-based recommendations are presented in…
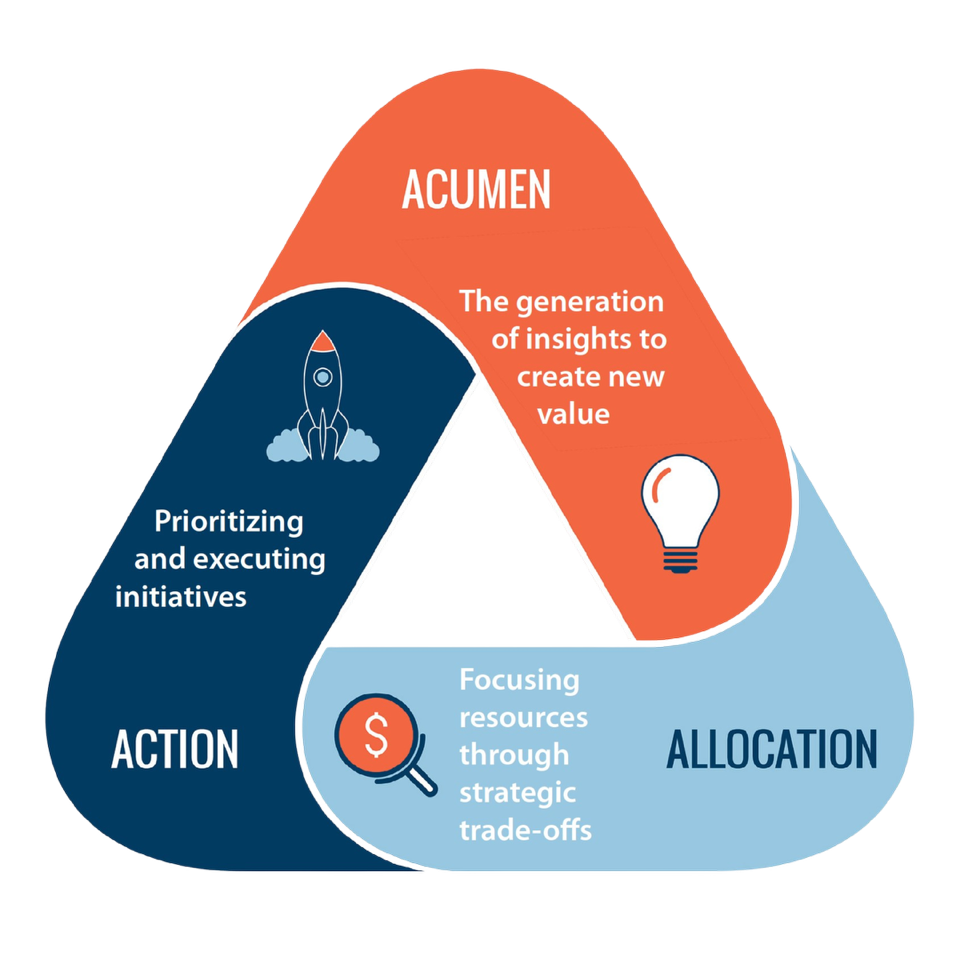
Takeaways • The 3A Strategic Thinking Framework and the GOST Framework are examples of tools that can help make the abstract process of strategic thinking more concrete for perioperative leaders. • Carving out dedicated time for planning and fostering strategic thinking in others are important to achieve optimal results. •…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…

Editor's Note Older adults face a significantly higher risk of complications including stroke and recurrent heart attacks if they undergo elective noncardiac surgeries soon after myocardial infarction, according to a University of Rochester study published October 30 in JAMA Surgery. According to a University announcement, current guidelines from the American…

Editor's Note Joint guidance from key US medical associations advises that most patients on GLP-1 weight-loss medications, such as semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro), may safely continue these drugs before surgery, HealthDay News reported October 31. Concerns had emerged about potential risks associated with these medications due to their…

Editor's Note New guidance from The Association for the Advancement of Medical Instrumentation (AAMI) addresses regulatory and safety concerns for hospitals using ethylene oxide (EO) for medical device sterilization—a pressing concern due to EO’s effectiveness but associated health risks. ANSI/AAMI ST58:2024, an update on chemical sterilization and high-level disinfection practices…