
Editor's Note Jasmine Hampton-Nicholson MSN, RN, CNOR, learned the value of a value analysis team (VAT) the hard way. “I thought that we had a very solid process for our value analysis team,” said the VP of perioperative services at Temple University Health System in a presentation at 2025 OR…
Editor's Note The US Food and Drug Administration (FDA) designated Baxter Healthcare Corporation’s recall of the Life2000 ventilator system as a Class 1, the most severe category indicating serious risk of injury or death. According to the agency’s February 5 announcement, a failed operation during manufacturing compromised the operation…

Editor’s Note At the Society of Thoracic Surgeons (STS) annual meeting, experts urged cardiac surgeons to become more engaged in tricuspid valve treatment before transcatheter options gain too much traction according to a January 26 report in MedPage Today. With new transcatheter devices gaining FDA approval and the Centers for…

Editor’s Note Healthcare systems are increasingly establishing long-term collaborations with medical technology companies with the goal of modernizing technology and streamlining operations through ongoing training and consultation services. Modern Healthcare reported the news January 24. Involving substantial financial investments, these collaborations redefine vendor relationships as more strategic and relational, the…

Editor's Note Analysts say proposed tariffs on imported goods could increase costs for approximately 75% of medical devices marketed in the United States, 69% of which are manufactured solely abroad, according to a January 20 article in Medical Product Outsourcing. Valued at $197.8 billion in 2023, the US medical device…

Editor's Note Although medical device shortages threaten all patients, a recent FDA announcement highlights particular risks for pediatric populations who require size-appropriate equipment. “From ventilators and neonatal breathing tubes to hemodialysis catheters, the scarcity of these critical devices poses a growing threat to patient care – especially for our youngest…

Editor's Note The US Food and Drug Administration (FDA) has issued new draft guidance January 6 providing recommendations improving the use of pulse oximeters and supporting the safety of AI-enabled medical devices, among other topics. Released January 6, draft guidance supporting the safety and effectiveness of AI-enabled devices would, if…
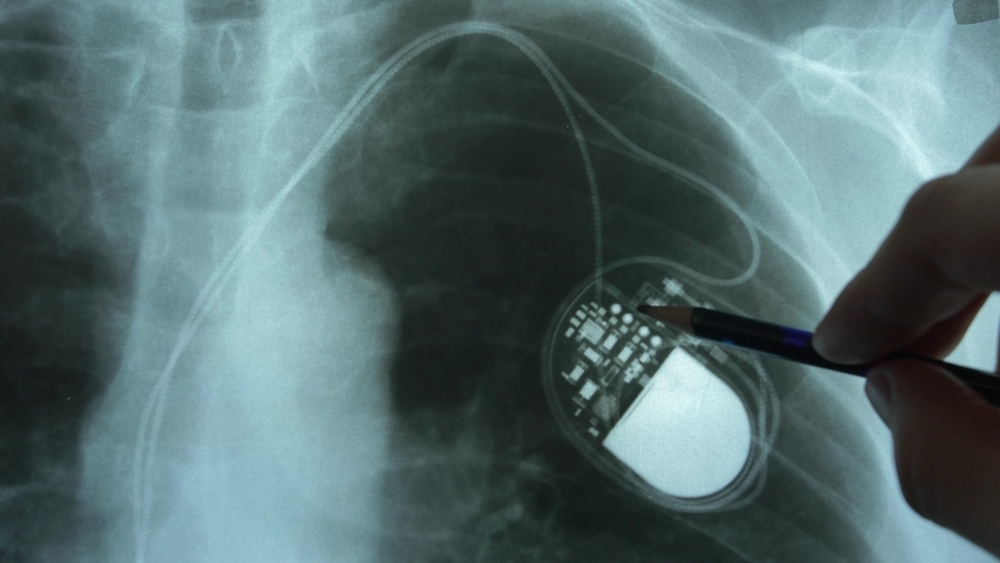
What happens when a surgeon uses the monopolar instrument set on 30-W coagulation mode to create an upper midline incision in a patient with a pacemaker? Pacemaker function is interrupted, causing a heart block that results in hemodynamic instability—or at least, this is what could happen without taking the necessary…

Editor's Note Balloon catheters for atrial fibrillation patients and implantable radiographic markers were the subjects of separate US Food and Drug Administration (FDA) Class 1 recalls—the most severe category indicating risk of serious injury or death—announced on December 18. The first recall involves Boston Scientific’s POLARx Cryoablation devices. Higher-than-anticipated reports…

Editor's Note Risk of delayed therapy and death related to Ivenix large-volume infusion pumps is prompting supplier Fresenius Kabi USA to pull a subset of the devices from the market, according to an early alert from The US Food and Drug Administration (FDA). Issued December 11, the early alert is…