Ambulatory surgery centers (ASCs) are taking on more high-acuity, same-day procedures than ever before. This growth is driven by evolving clinical protocols, cost-conscious reimbursement strategies, and expanded capabilities in outpatient care. Specialties like orthopedics, spine, ophthalmology, and cardiovascular care are moving more complex cases—and the implants that come with them—out…

Editor's Note A new brain implant could one day restore voices to those who can no longer speak, the Associated Press (AP) reported March 31. As detailed in the article, researchers have successfully tested the experimental brain-computer interface (BCI) on a 47-year-old woman with quadriplegia who lost the ability to…

Editor's Note Arthroplasty implants may release metals that accumulate in the central nervous system (CNS), potentially contributing to neurotoxic effects, according to a study published March 28 in JAMA Network Open. Researchers found that patients with large joint replacements had significantly higher levels of cobalt, chromium, titanium, niobium, and zirconium…

Editor's Note Miniaturized pacemakers implanted in neonates and infants have demonstrated reliable performance for up to two years, with no unexpected device failures, according to a March 11 article in Healio. The research, published in Circulation: Arrhythmia and Electrophysiology, suggests that these modified pacemakers could offer a viable alternative for…

Editor's Note An Australian man lived for 100 days with an artificial titanium heart, the longest duration recorded for a patient using the device, according to a March 12 report from CNN. The breakthrough marks a significant step toward using total artificial hearts as a long-term solution for patients with…

Editor’s Note The US Food and Drug Administration (FDA) has designated Boston Scientific Corporation’s recall of Accolade Pacemaker devices a Class 1, the most severe category indicating serious risk of injury or death. According to the agency’s February 21 announcement, the recall was motivated by a manufacturing issue that could…

Editor's Note In this session, Deb Yoder, MHA, BSN, RN, CNOR, CASC, vice president of facility development, Compass Surgery Partners, provided a comprehensive overview of selecting, implementing, and managing service lines in ambulatory surgery centers (ASCs) while considering factors such as block time utilization, staff readiness, equipment needs, financial viability,…
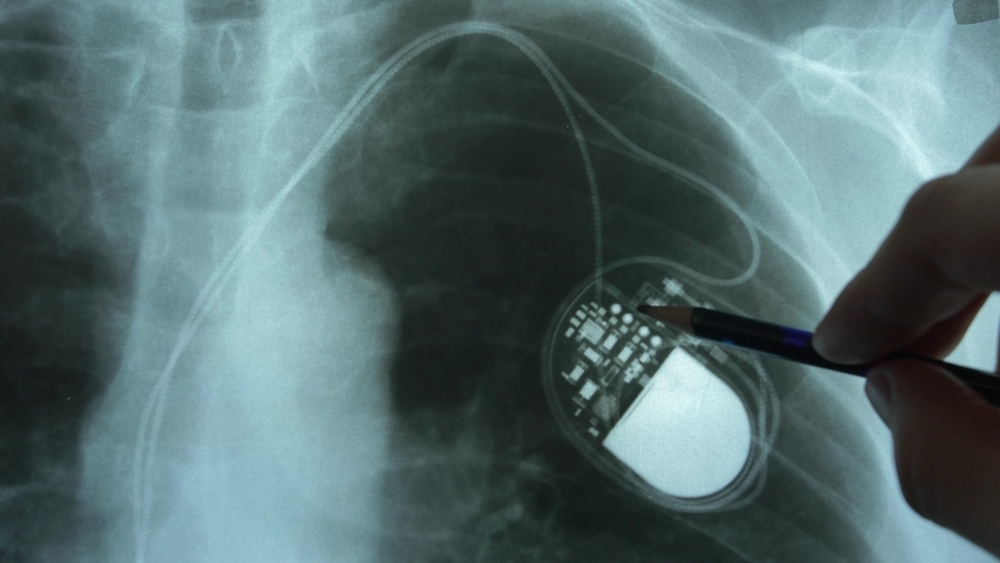
What happens when a surgeon uses the monopolar instrument set on 30-W coagulation mode to create an upper midline incision in a patient with a pacemaker? Pacemaker function is interrupted, causing a heart block that results in hemodynamic instability—or at least, this is what could happen without taking the necessary…

Editor's Note The medical 3D printing market is expected to double from $2 billion in 2022 to $4 billion by 2026, driven by customization, lower costs, and quick turnarounds, according to analysis from GlobalData. In a July 24 report on the analysis, Medical Device Network outlined this growth as well…

Editor's Note Mini-slings for stress urinary incontinence (SUI) are similarly effective to mid-urethral slings over a 36-month timeframe, according to an April 11 announcement from the US Food & Drug Administration (FDA). FDA reached this conclusion after an in-depth, systematic literature review of post-market surveillance (“522”) studies required last year…