Editor's Note The Food and Drug Administration (FDA) on August 30 issued a Safety Alert reminding healthcare providers that using thermal regulating systems, including forced air systems, during surgical procedures has been shown to result in less bleeding, faster recovery times, and decreased risk of infections for patients. The FDA…

Editor's Note At least 23 hospitals in the Houston area affected by flooding from Hurricane Harvey have moved patients to other facilities, and another 25 are considered vulnerable to flooding or shortages of staff and supplies, the August 29 Houston Chronicle reports. The full tally of evacuated patients was not…

Editor's Note The Joint Commission has issued a new R3 Report to help accredited hospitals better understand and comply with its new and revised pain management standards, which are effective January 1, 2018. This R3 Report provides in-depth rationale, references, and evidence used in the development of the new and…
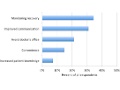
Editor's Note This survey of 800 patients identified a range of potential barriers and benefits to using mobile health technology to enhance recovery after surgery. Potential barriers included: protecting personal health information technology effectiveness and failure preference for face-to-face interaction with their surgeons level of effort required ability of older…
Editor's Note The Food and Drug Administration (FDA) announced on August 29 that it had approved a firmware update that is intended as a recall, specifically a corrective action, to reduce the risk of patient harm due to cybersecurity vulnerabilities for certain Abbott (formerly St Jude Medical) implantable cardiac pacemakers.…

Editor's Note Postoperative pain trajectories can identify patients at risk for 30-day readmissions and emergency department (ED) visits, and are not mediated by postdischarge complications, this study finds. In this analysis of 211,231 surgical procedures (45.4% orthopedic, 37.0% general, and 17.6% vascular), the 30-day unplanned readmission rate was 10.8%, and…
Editor's Note The Food and Drug Administration (FDA) on August 25 announced a recall correction to the instructions for use by Cook Medical (Bloomington, Indiana) for its Zenith Alpha thoracic endovascular graft. This correction removed the indication for blunt thoracic aortic injury (BTAI) because Cook has received an increase in…

Editor's Note Flooding from Hurricane Harvey is pushing some Houston hospitals to close or evacuate, the August 27 Modern Healthcare reports. Baylor College of Medicine and clinics closed Sunday, August 27, as did all Texas Children’s Hospital’s pediatrics practices and urgent care locations. The University of Texas MD Anderson Cancer…

Editor's Note This meta-analysis found that patients at higher risk for obstructive sleep apnea (OSA), as determined by preoperative STOP-Bang screening, had a higher risk of postoperative adverse events and longer length of hospital stay, compared with lower risk-OSA patients. The analysis included 10 studies with 23,609 patients (7,877 high-risk…

Editor's Note In this analysis of patient falls from OR and procedure tables during anesthesia care, researchers queried two independent closed claims databases. They identified 21 claims (1.2% of cases) in the American Society of Anesthesiologists (ASA) Closed Claims Project and 0.07% of cases in the secure records of a…