Editor's Note The Food and Drug Administration (FDA) on August 16 classified the recall by Cook Medical Inc (Bloomington, Indiana) of its Zenith Alpha Thoracic Endovascular Graft as Class I, the most serious. Cook Medical is aware of reported cases where the graft became blocked or closed with blood clots…

Editor's Note In this multicenter study, on-pump coronary artery bypass grafting (CABG) led to significantly higher rates of 5-year survival and event-free survival than off-pump CABG. From 2002 to 2007, a total of 2,203 patients at 18 medical centers were randomly assigned to undergo either on-pump (1,099 patients) or off-…

Editor's Note The Joint Commission on August 16 announced a new Quick Safety that focuses on noise and distractions in the OR and how they can make it difficult to hear and discern information or communicate effectively. ORs are prone to high levels of noise, which can be distracting and…

Editor's Note When colorectal surgical patients, who were given a single dose of antibiotic before surgery and re-dosing if the procedure lasted longer, were compared to patients given additional antibiotics for 24 hours postoperatively, infection rates were identical, this study finds. A total of 965 patients were included in this…
Editor's Note The Food and Drug Administration (FDA) on August 10 issued a Safety Alert to update healthcare providers on five reports of unanticipated deaths that have occurred from 2016 to the present in patients with liquid-filled intragastric balloon systems used to treat obesity. Four reports involve the Orbera Intragastric…

Editor's Note In this study, patients diagnosed on the day of surgery as moderate to high risk for obstructive sleep apnea (OSA) had similar rates of adverse respiratory events (ie, perioperative hypoxemia and difficult airway management) as patients who had been diagnosed with OSA previously. However, those diagnosed with OSA…

Editor's Note Adding cognitive assessment to frailty assessment predicted poor postoperative outcomes and survival in frail patients better than either measurement alone, this study finds. The study included 330 patients having major surgery who were assessed with a four-level composite frailty scoring system, which was created by combining the Fried…
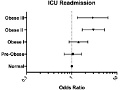
Editor's Note After cardiac surgery, obese patients required significantly more ICU resources and longer recovery times, resulting in more expensive, labor-intensive care, this study finds. Of 5,365 patients included in the analysis, 1,948 were classified as obese. Patients with greater obesity were: four times more likely to have longer time…

Editor's Note Cybersecurity experts are warning that Ransomware 2.0 is coming, and healthcare needs to be prepared, the August 8 Healthcare IT News reports. Ransomware 2.0 combines ransomware with a worm, which allows it to move laterally or across internal and external networks. Because the next wave of attacks will…
Editor's Note Researchers have released a surgical technical evidence review for colorectal surgery (published online August 7 in the Journal of the American College of Surgeons) as the first step in the Agency for Healthcare Research and Quality’s Safety Program for Improving Surgical Care and Recovery, a nationwide program to…