Editor's Note Hospitals with rectal cancer programs may now earn accreditation from the new National Accreditation Program for Rectal Cancer (NAPRC), the American College of Surgeons (ACS) announced on June 21. NAPRC is based on successful international models that have resulted in better outcomes and emphasize a multidisciplinary team approach…
Editor's Note The Food and Drug Administration (FDA) on June 21 announced the recall by Vascular Solutions (Maple Grove, Minnesota) of its Venture Catheters. The recall was initiated because there is a risk of the catheter tip splitting or separating during use and entering the patient’s bloodstream. This can result…

Editor's Note In this single institution study, the most common reasons for unplanned return to the OR (uROR) were infection and hemorrhage. However, the researchers found that a large number of cases were incorrectly classified as uROR, when they were instead planned reoperations without adequate documentation. Using uROR as reported…

Editor's Note The Joint Commission announced on June 21 a redesign of its Evidence of Standards Compliance (ESC) form. The form was redesigned to help organizations focus on describing the critical aspects of corrective actions they have taken to resolve Requirements for Improvement, and to help ensure sustainability of those…
Editor's Note The Food and Drug Administration (FDA) on June 16 announced the recall by Alvogen/Hospira Inc, a Pfizer company, of seven lots of Clindamycin Injection USP ADD-Vantage Vials. The recall was initiated because microbial growth was detected during a routine simulation of the manufacturing process, which represents the potential…
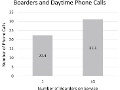
Editor's Note Surgical ICU patients boarding in alternative ICUs because of overcrowding are often seen at the end of rounds, receive fewer face-to-face assessments from physicians, and are given less bedside attention by ICU provider teams, this study finds. The researchers found that: caregivers spent about 16% less time on…
Editor's Note The Food and Drug Administration (FDA) on June 19 announced the recall by Maquet/Datascope (Fairfield, New Jersey) of its System CS100, CS100i, and CS300 Intra-Aortic Balloon Pumps. The recall also applies to System 98 or System 98XT IABP that was converted to a CS100i or CS 300 IABP.…
Keeping a close eye on implants that are opened and not used is one way OR leaders can track practices that add significantly to costs. But what about blood products? Blood taken to the OR and not used also can be costly. Although an individual unit of blood doesn't compare…
Researchers at the University of Michigan (U-M), Ann Arbor, have invented a new surgical instrument with the goal of addressing a vast, unmet need in minimally invasive surgery. For less than a thousand dollars, this platform technology—currently being commercialized by the start-up FlexDex Surgical—offers capabilities similar to those of the…
Healthcare providers and their patients have benefited from practices borrowed from other industries—for example, the checklists and time-outs used in aviation or the Lean management principles used in manufacturing. Applying lessons learned in the entertainment industry to the hospital setting may seem like a stretch, but Dennis Snow will explore…