
Editor's Note Research reveals nearly 10% of patients treated with prescription opioid painkillers develop opioid dependency or opioid use disorder (OUD), Healio reported on August 13. Additionally, nearly 30% of patients exhibit signs and symptoms indicating potential OUD. Originally published in the journal Addiction, the data are from a systematic…
Editor's Note Human trials may begin soon on patients in the UK using tiny, folding brain implants that could improve epilepsy surgery, according to an article published August 12 in The Telegraph. Developed by scientists at Oxford and Cambridge, who published their research in the journal Nature Communications, the implants…
Every year, OR Manager shines a light on staffing issues via the Salary/Career Survey. In this issue, two articles take a careful look at the career and profile of the perioperative leader in both inpatient and outpatient settings. Next month, two more articles will do the same with salary trends…

Asking who, what, why, when, where, and how—otherwise known as the “5 Ws and an H”— is a time-tested way for writers and researchers to ensure comprehensive coverage of any topic. Here, we apply this framework from the perspective of sterile processing department (SPD) professionals seeking to start a water…

Human trafficking (HT) is a global public health crisis and one of the fastest growing criminal enterprises that grosses hundreds of billions of dollars annually, all of which are tax-free profits made off the sale of human cargo. And yet, research shows the problem is poorly understood or recognized among…
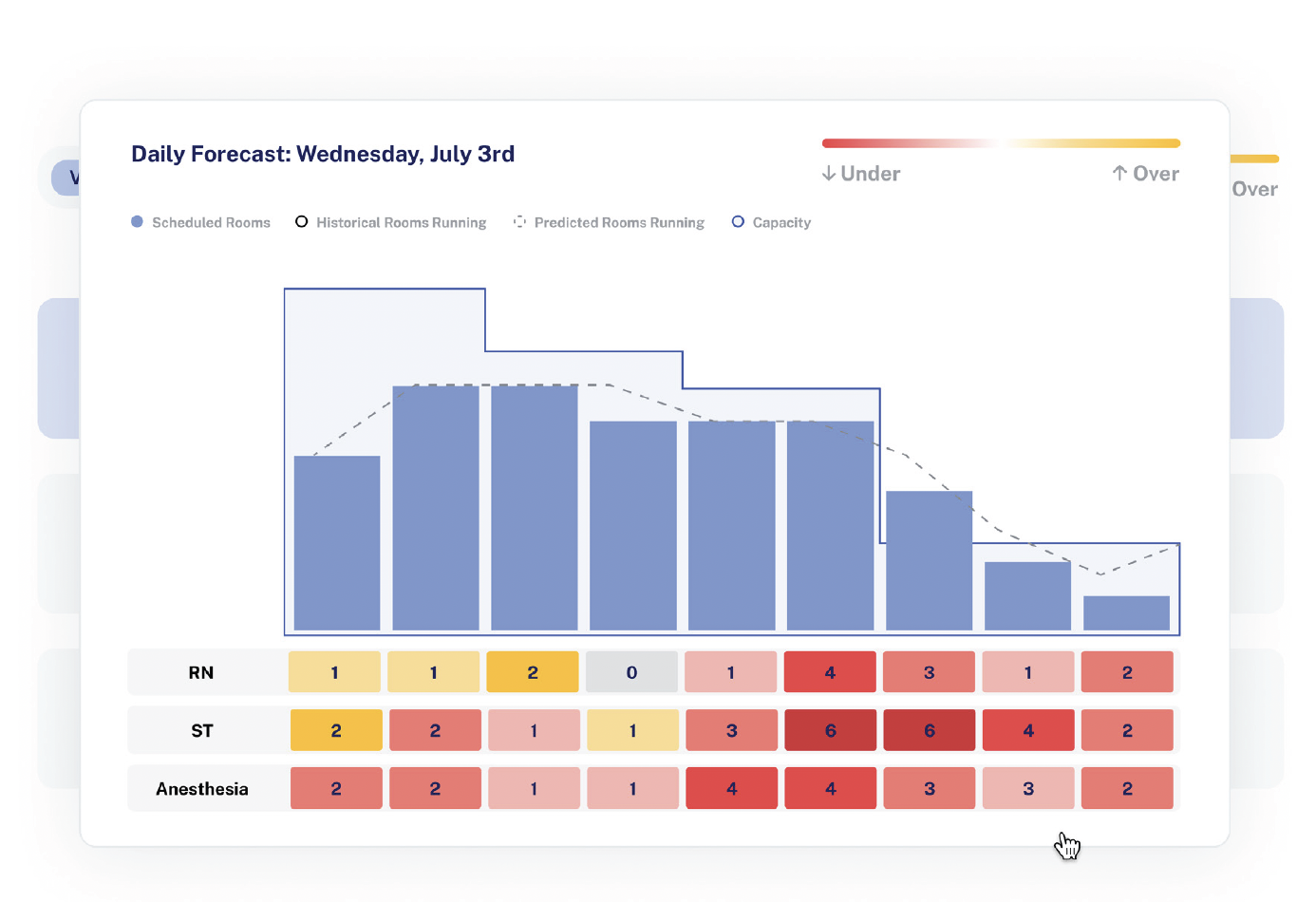
Perioperative leaders face mounting pressures to optimize resources, reduce costs, and improve patient outcomes. However, one challenge stands out among the rest: OR staffing shortages. According to a November/December survey conducted by LeanTaaS in collaboration with OR Manager, staff recruitment and retention is a top priority for OR leaders this…

Editor's Note A 13-year study at US Veterans Affairs (VA) medical centers found a decline in both hospital-associated infections (HAIs) and antimicrobial resistance for common pathogens, MedPage Today August 15 reports. From 2007 to 2019, the overall infection rate of nine pathogens decreased, with an average annual percentage change (AAPC)…

Editor's Note A 2016 recall issued for the Abbott MitraClip cardiac device highlighted potential safety concerns, but instead of removing the product from the market, Abbott and the Food and Drug Administration (FDA) allowed continued use with revised instructions and additional training for doctors. This approach reflects a broader trend…

Editor's Note Effective waste segregation and adopting a circular economy approach can significantly reduce environmental impact of incinerating hospital waste incorrectly classified as hazardous, according to a narrative review published August 19 in the Medical Journal of Australia. However, surgeons' concerns about patient safety and insufficient systemic policies can hinder…

Editor's Note The “surgical pause”—a means of validating whether surgery is truly safe for patients deemed to be “frail” before starting a procedure—significantly reduces mortality rates and is changing practices at more than 50 Veterans Administration (VA) hospitals, the Pittsburgh Post-Gazette reported August 18. Developed by VA surgeons Daniel Hall…