
Editor's Note In a recent study, an artificial intelligence (AI) system detected more clinically significant prostate cancers and fewer indolent cancers than human radiologists reading MRIs, MedPage today reported June 13. The MedPage report covers a study published in Lancet Oncology that, according to researchers, “provided evidence that AI systems,…

Editor's Note COVID infections are rising nationally, with cases increasing in 39 states and none showing a decline, NBC news reported June 24. The Centers for Disease Control and Prevention (CDC) tracks Covid transmission through emergency department visits, which, along with deaths and hospitalizations, have recently increased, NBC reports. California…

Editor's Note A June 24 advisory from the FBI and Department of Health and Human Services warns healthcare organizations about attempts to steal payments through phishing and social engineering tactics, according to a post from the American Hospital Association (AHA). The attackers target employee email accounts to access login information…

Editor's Note Nearly half of hospital executives report that their hospitals are not fully prepared to cope with patient volumes, Becker’s Hospital Review reported June 13. Citing the June 12 Hospital Operations Outlook Survey from FTI Consulting, Becker’s reports that nursing and mental and behavior health specialists represent the greatest…

Editor's Note CMS hospital star ratings may not be a reliable tool for assessing surgical quality, according to a study published June 18 in JAMA Surgery. Researchers acknowledge that higher ratings are generally associated with improved postoperative outcomes, including fewer complications and lower 30-day mortality rates. However, as reported by…

Editor's Note In a first for Northwestern Medicine, surgeons performed a kidney transplant on an awake patient, CBS News reported June 24. John Nicholas, 28, of Chicago, experienced no pain during the May 24 procedure, in which he received an organ from a childhood friend. He was discharged the next…

Editor's Note Qilin, a ransomware group based in Russia, claimed responsibility for a cyberattack against pathology services provider Synnovis that paralyzed London Hospitals and is now requesting $50 million, Becker’s Health IT reported June 20. Citing a report from Bloomberg, the article notes that the attack disrupted services at London-based hospitals…

Editor's Note Change Healthcare has started to notify health care providers about patient data stolen in the February cyberattack and announced plans to mail affected individuals as well. A unit of UnitedHealth Group, the organization issued the update June 20. “CHC is providing this notice now to help individuals understand…
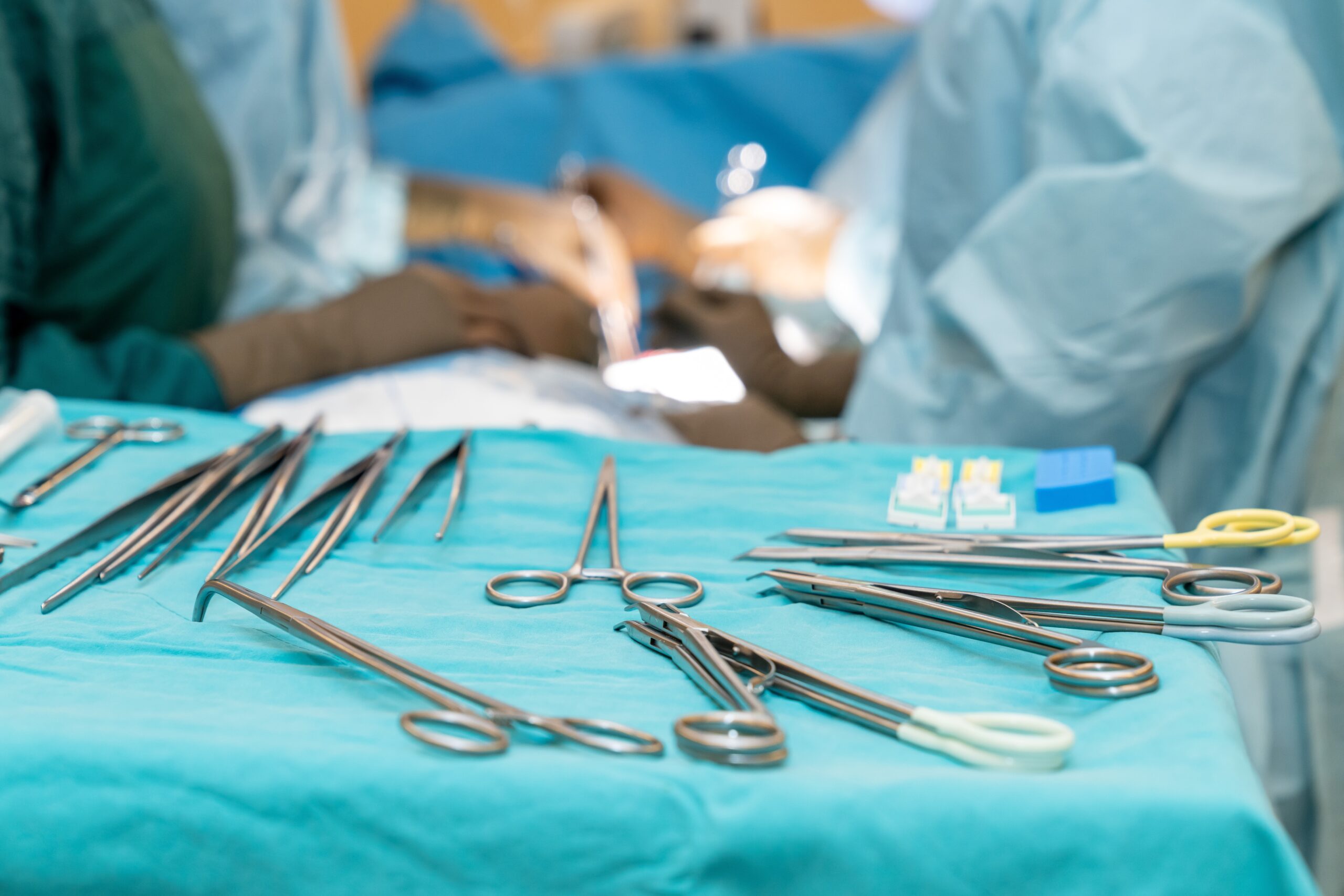
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…

When it comes to the adoption of artificial intelligence (AI) in medicine, radiology is leading the charge. As of May 13, 2024, the US Food and Drug Administration (FDA) had approved nearly 900 AI- and machine learning (ML)-enabled devices, and the vast majority of them are in radiology. One example…