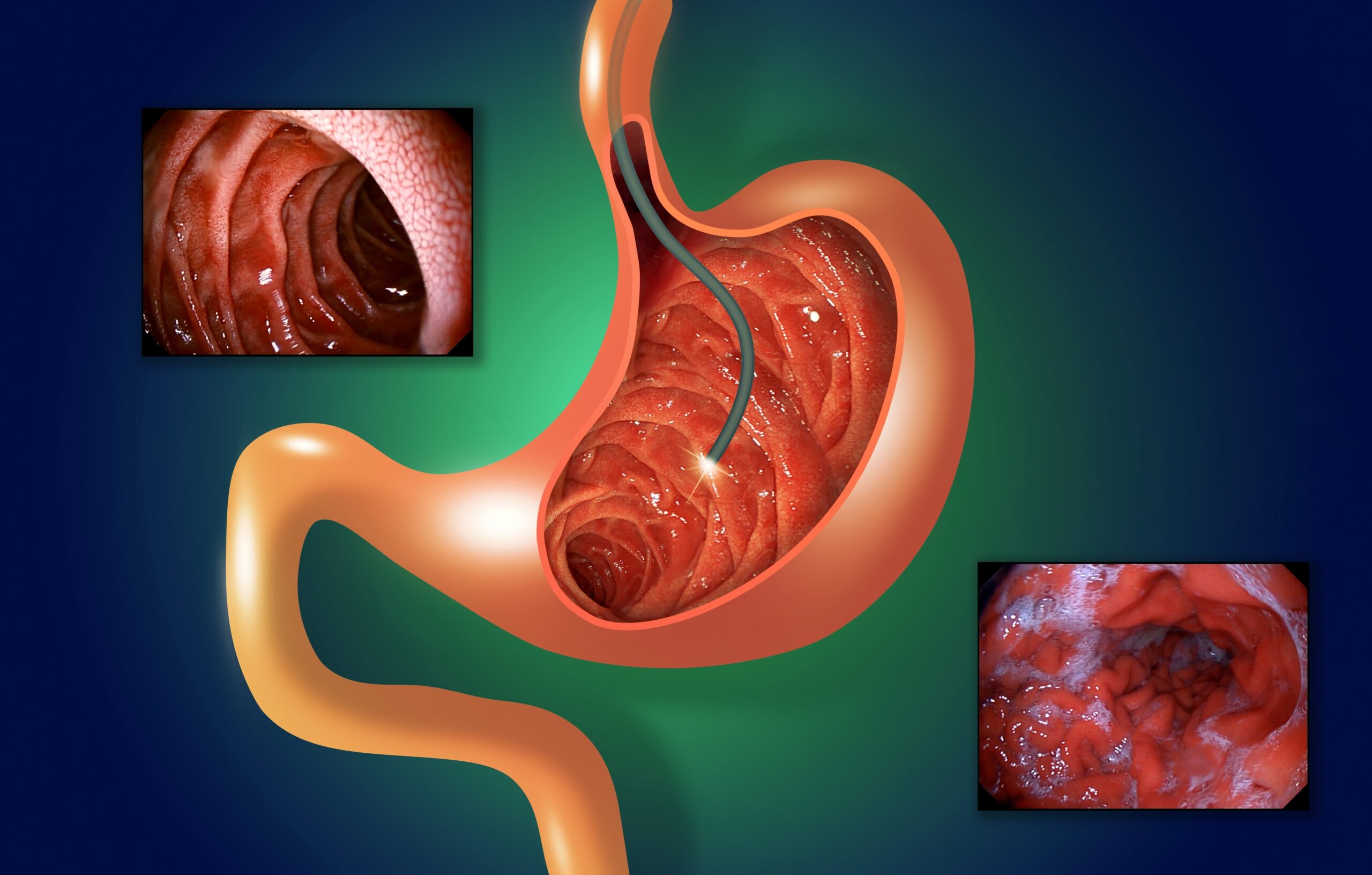
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…

Rural hospitals in the US have been facing a prolonged, multifaceted crisis. The literature presents several reasons for why healthcare facilities in rural areas struggle, including shrinking budgets, rising chronic illness and public health issues like addiction and obesity, poor telehealth and broadband access, aging populations, deteriorating mental health, and…

Editor's Note Roux-en-Y gastric bypass surgery kept type 2 diabetes in remission for up to 15 years and maintained most of the weight loss for up to 20 years in a long-term study, according to a June 12 report in SciTech Daily. Presented the same day at The American Society…

Editor's Note Surgeons are more likely to be reported for unprofessional behavior than any other category of physician, and pediatric specialists are least likely, according to a study published June 6 in Jama Network Open. Based on data from the Center for Patient and Professional Advocacy's Coworker Observation Reporting System…

Editor's Note Substituting lower-wage staff for registered nurses leads to additional patient deaths, the Washington Post reported on June 15. The article focuses on a study published in the journal Medical Care, noting that the new research coincides with a nationwide shortage of RNs and “reports of widespread burnout.” Researchers…
Editor's Note A pair of immunotherapy drugs administered before surgery significantly diminished tumor size without serious safety concerns in patients with mismatch repair-deficient (dMMR) colorectal cancer, according to a study published in the New England Journal of Medicine. Healthline reported the news June 8. Constituting 10-15% of cases, dMMR cancer…

Editor's Note Surgeons and surgical trainees who are female or from minority racial or ethnic backgrounds report higher levels of negative emotions and self-doubt after adverse events, according to a recent study in JAMA Network Open. According to a June 5 report in MedPage Today, the single-site, mixed-methods study found…

Editor's Note Robotic liver surgery can be performed safely as an outpatient procedure, according to findings from the City of Hope cancer research organization in Duarte, California. According to a June 10 press release, the study analyzed data of 307 patients who underwent outpatient robotic liver surgery (defined as requiring…

Editor's Note Bariatric surgery provides longer-lasting, more effective weight loss than GLP-1 receptor agonists and lifestyle interventions, according to systematic reviews of medical literature from 2020 to 2024. Medical Xpress reported the news June 11. Presented at the American Society for Metabolic and Bariatric Surgery (ASMBS) 2024 Annual Scientific Meeting,…

Editor's Note Ascension has restored electronic health records (EHRs) throughout all hospitals and clinics nationwide, according to a June 14 update from the St. Louis-based health system. "Clinical workflow in our hospitals and clinics will function similarly to the way it did prior to the ransomware attack," the statement reads,…