
Editor's Note The US Food & Drug Administration has designated Medline Industries’ recall of the Sub-G Endotracheal Tube with Subglottic Suction, which is used to keep a patients’ airway open, as a class 1, the most severe category indicating risk of serious injury or death. According to the agency’s June…

Editor's Note The US Food & Drug Administration (FDA) has classified Medtronic’s recall of its StealthStation S8 software as a Class I, the most severe category indicating risk of serious injury or death. The StealthStation System with StealthStation Cranial software is intended as an aid for locating anatomical structures during…

Editor's Note The US Food and Drug Administration (FDA) has designated OptumHealth Care Solutions’ recall of the Nimbus II ambulatory infusion pumps a class 1, the most severe category indicating risk of serious injury or death. According to the agency’s May 30 announcement, the company recalled the pumps in direct…
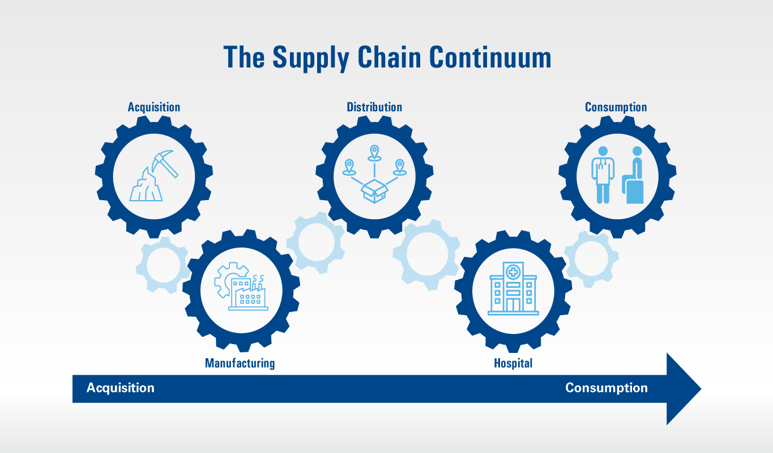
More than 4 years after personal protective equipment production and inventories crashed amid the global COVID-19 pandemic, the “new normal” in healthcare supply chains seems to be “uncertainty.” Although hospital margins are improving and patient volumes are trending upward, “stress fractures continue to remain in place,” says Michael Schiller, CMRP,…

Editor's Note The US Food and Drug Administration (FDA) has designated the recall of Hologic Inc.’s BioZorb Marker a class 1, indicating a risk of serious injury or death. BioZorb is an implantable radiographic marker used to mark soft tissue (such as breast tissue) for future medical procedures. Provided sterile…

Editor's Note The lack of available hearts for transplantation combined with the recent recall of Abbott’s HeartMate 3 left ventricular assist device (LVAD) “makes the current therapy landscape for heart failure much more dire,” according to a May 20 report in Medical Device Network. The recall of the device, which…

Editor's Note The number of US Food and Drug Administration (FDA) Class 1 recalls—the most serious classification—for medical devices has been trending upwards. Regulatory News, a publication of the Regulatory Affairs Professional Society (RAPS), reported the news April 25. According to the report, Anne Reid, program director of the Office…

Editor's Note Microscopic stainless steel debris on the insides of biopsy needles prompted the FDA to issue a class 1 recall—indicating risk of death or serious injury—for Elekta Instrument’s Disposable Biopsy Needle Kit, which is used with the Leksell Stereotactic System for brain tissue sampling during neurosurgery, the disposable biopsy…

Editor's Note A January complaint about inefficient packaging for joint replacement products has prompted the manufacturer to recall certain knee and shoulder system devices after initially declining to take the products off the market, according to an April 23 report in Health Exec. The manufacturer, Exactech, is now recalling a…

Editor's Note The US Food and Drug Administration has designated DeRoyal Industries’ recall of GeoMed custom tracecarts a class 1, the most serious type of recall indicating a risk of serious injury or death. According to the April 24 FDA notice, the recall is due to sterility concerns with the…