For decades, ambulatory surgery centers (ASCs) have shown their ability to deliver high-quality surgical care at substantially lower cost than hospital outpatient departments (HOPDs). ASCs achieve these savings through leaner operations, streamlined staffing models, and specialty-focused efficiencies, not by compromising safety or outcomes. Studies consistently highlight procedures performed in ASCs…

As we celebrate National ASC Month, it is worth recognizing how central ambulatory surgery centers (ASCs) have become to modern healthcare. Today, there are more than 12,000 ASCs across the US, including over 6,500 Medicare-certified facilities operating some 18,800 surgical suites. With over 80% of all surgical procedures being performed…

Editor's Note The FDA has issued a Class 1 recall—the most severe category indicating risk of serious injury or death—for Draeger Filter SafeStar 90 Plus (MP05785), Filter SafeStar 55 Plus (MP05790), Filter SafeStar 60A Plus (MP05795), and Filter/HME TwinStar HEPA Plus (MP05801) breathing system filters, affecting all lot numbers. Draeger…

Editor's Note Poor planning and rushed decisions derail too many ambulatory surgery centers (ASCs) before they open their doors. In a recent blog post, ASC consultant Emily Spooner outlined the top five errors commonly made during ASC development, offering targeted guidance on how to avoid them. According to the post,…

Editor's Note Healthcare providers could experience significant cash flow and operational changes under a new voluntary pilot program that fundamentally alters how the nation's second-largest drug payment program operates, according to an August 11 article in Modern Healthcare. As detailed in the article, The Health Resources and Services Administration announced…

Editor's Note The US Food and Drug Administration (FDA) is requiring safety labeling changes for all opioid pain medications to better emphasize and explain the risks associated with long-term use, according to a July 31 announcement. These changes follow a May advisory committee meeting where the agency reviewed data on…

Editor's Note Hundreds of urban hospitals have obtained dual urban-rural Medicare classifications since a 2016 policy change, enabling them to qualify for reimbursement programs intended for rural providers. Fierce Healthcare reported the news August 4. As detailed in the article, a study published in Health Affairs by Johns Hopkins and…

For an ambulatory surgery center (ASC), earning accreditation can be more than a mark of excellence. The Accreditation Association for Ambulatory Health Care (AAAHC) offers Medicare Deemed Status Accreditation, eliminating the need for separate surveys to certify compliance with the Conditions of Participation (CoPs) required by the Centers for Medicare…

Editor’s Note: This page is a companion piece to the main article, How ASCs ace the AAAHC accreditation survey. The posts below cover the latest version of the handbook from the Accreditation Association for Ambulatory Health Care (AAAHC) and advice on delineation of privileges. Navigating the new handbook The latest…
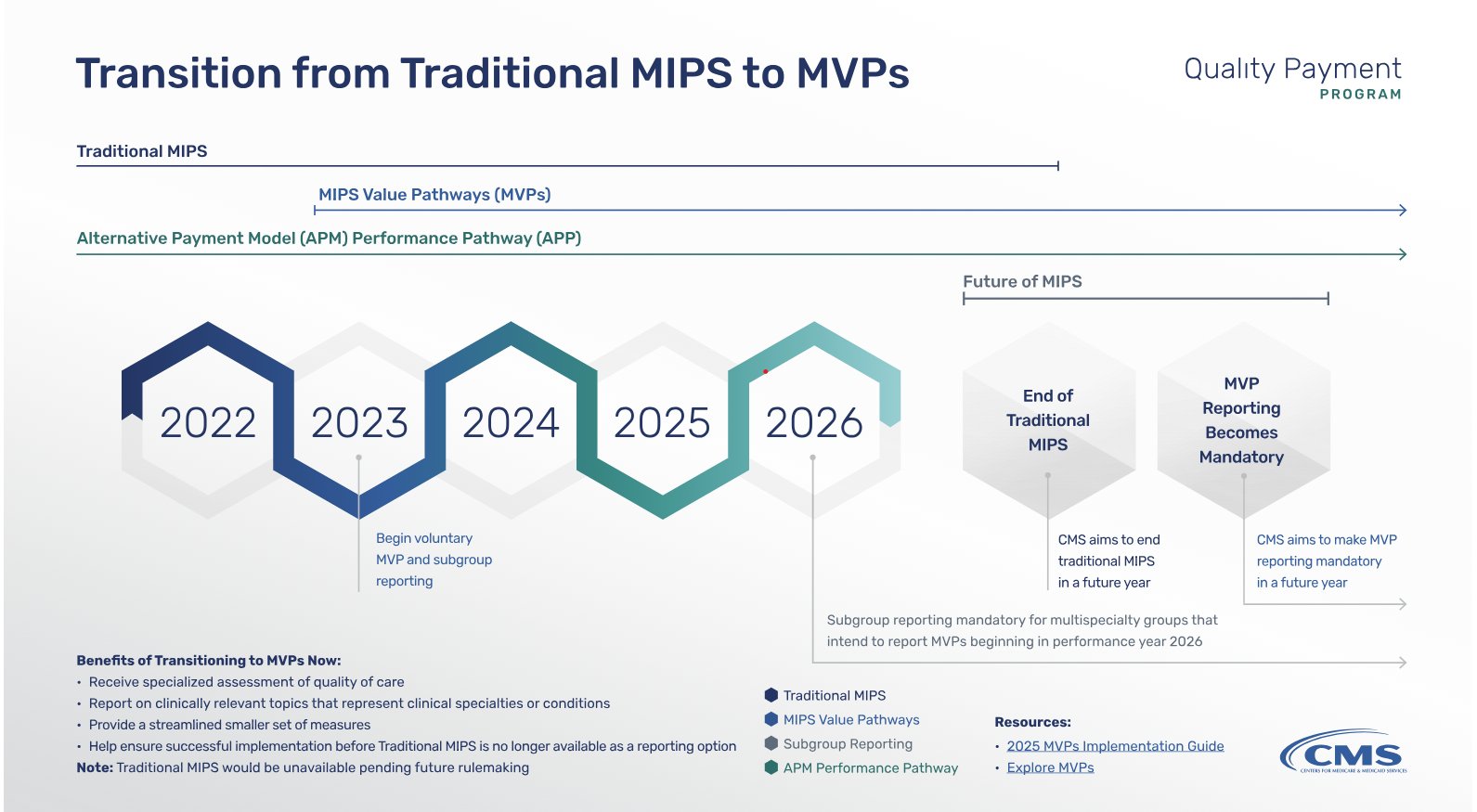
In July 2025, the Centers for Medicare & Medicaid Services (CMS) proposed sweeping changes to the Medicare Physician Fee Schedule for calendar year 2026. Among the most impactful updates is the launch of the Ambulatory Specialty Model (ASM)—a mandatory value-based payment program focused on heart failure and low back pain.…