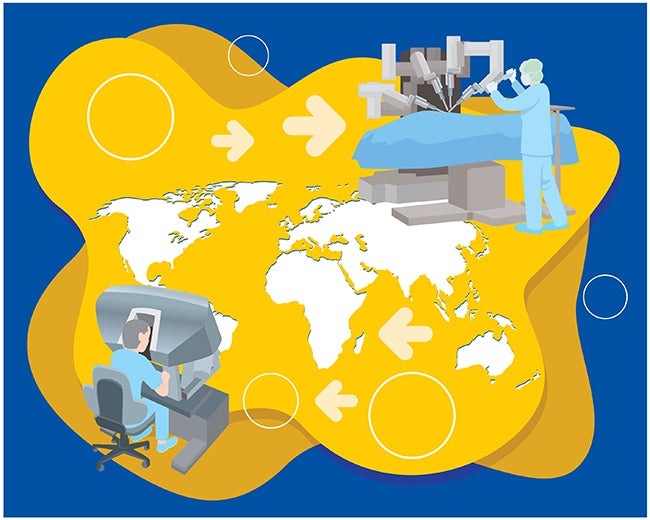
Remote surgery has come a long way since the first-ever case in 2001, when a surgeon in New York City operated on a patient in Strasbourg, France. No longer a product of science fiction, telesurgery’s advance promises to change—and save—countless lives, from patients in remote areas to those in warzones…
Editor's Note Nonprofit hospitals, which are legally required to provide free or discounted care to qualifying patients, attempt to collect hundreds of millions of dollars from low-income patients annually while receiving significant tax breaks meant to ensure affordable care, according to a May 12 article from CBS News. As…

Editor's Note The Trump administration has dismantled the federal committee responsible for shaping national infection prevention standards in hospitals, sparking concern among healthcare experts over future preparedness, NBC News reported May 6. According to the article, the Centers for Disease Control and Prevention (CDC) informed members of the Healthcare Infection…

Takeaways • US surgeons have no mandated retirement age. According to the Aging Surgeon Program, “a patient death or serious negative event are currently the only things that prompt action to prevent a surgeon from practicing.” • Research on aging-related decline is clear, but nuanced, showing rates and scope vary…

Editor's Note State attorneys general from 20 states filed suit to block major restructuring and layoffs at the Department of Health and Human Services (HHS), alleging the actions bypass Congress, violate federal law, and endanger public health. As detailed in a May 5 report from Fierce Healthcare, the lawsuit seeks…

Editor's Note At the 2025 Ambulatory Surgery Center Association (ASCA) conference, discussion of legislative priorities extended beyond educational sessions. Thanks to a visit from the ranking lawmaker of the congressional subcommittee responsible for most healthcare policymaking, attendees also got first-hand insight into the latest negotiations on Capitol Hill. US Representative…

Editor's Note A thoughtful, operations-driven approach to ambulatory surgery center (ASC) facility design can prevent major delays, costly change orders, and inefficient workflows down the line, according to a May 2 presentation from Akshay Tavkar, MBA, CMPE, CASC, principal and managing director of Skyline Healthcare Solutions, LLC, at the Ambulatory…

Editor's Note Sudden, unexpected shutdown and restart prompted the US Food and Drug Administration (FDA) to designate a class 1 recall—the most severe category reserved for serious risk of injury or death—for Abbot’s HeartMate Mobile Power Unit, which powers system controllers for the HeartMate 3 Left Ventricular Assist System (LVAS)…

Editor's Note Aortic root cannulas from Medtronic are the subject of the latest US Food and Drug Administration (FDA) class 1 recall, the most serious category reserved for risk of injury or death. Affected products include the DLP Aortic Root Cannula, MiAR Cannula, and DLP Aortic Root Cannula with Vent…

Editor's Note The bipartisan Medical Supply Chain Resiliency Act would grant the president expanded authority to negotiate trade deals and modify tariffs on medical goods, according to a March 14 report from Fierce Healthcare. The bill, backed by pharmaceutical and healthcare industry groups, aims to strengthen the US medical supply…