
Editor's Note The US Food and Drug Administration (FDA) has designated recent medical device recalls involving GE Healthcare’s Carestation anesthesia system, Medtronic aortic root cannula systems, Zoll Circulation’s AutoPulse NXT Resuscitation System, and Medtronic’s Bravo CF Capsule Delivery Devices as Class 1, the most severe category indicating serious risk of…

Editor's Note The Joint Commission and the Coalition for Health AI (CHAI) are partnering to set national standards for responsible artificial intelligence (AI) use in healthcare, according to a June 11 announcement from the organizations. This effort will deliver AI implementation guidance, tools, and a certification program to over 80%…

Editor's Note CMS has rescinded a 2022 guidance that protected clinicians providing emergency abortion care under the Emergency Medical Treatment & Labor Act (EMTALA), removing a key federal safeguard for providers in states with abortion restrictions, according to a June 2 article in Becker’s Hospital Review. The guidance, originally issued…

The wave of new legal requirements for surgical smoke evacuation across the country has given OR leaders a crash course in how to act on any new legislation. Based on this experience, complying with other new and pending laws will put these skills to the test. Major hurdles are likely…

Editor's Note The Trump administration’s proposed fiscal year 2026 budget calls for slashing the Department of Health and Human Services (HHS) discretionary budget by $32 billion, a nearly 25% cut that brings the total to $95 billion. Fierce Healthcare reported the news June 2. Although many of the proposed cuts…

Editor's Note An AI-powered wearable camera has achieved 99.6% accuracy in detecting potentially deadly drug mix-ups, including in the OR, NBC News reported May 25. Developed by Kelly Michaelsen, MD, at UW Medicine, the smart glasses scan medication labels in real time, alerting anesthesia providers to syringe-vial mismatches before drugs…
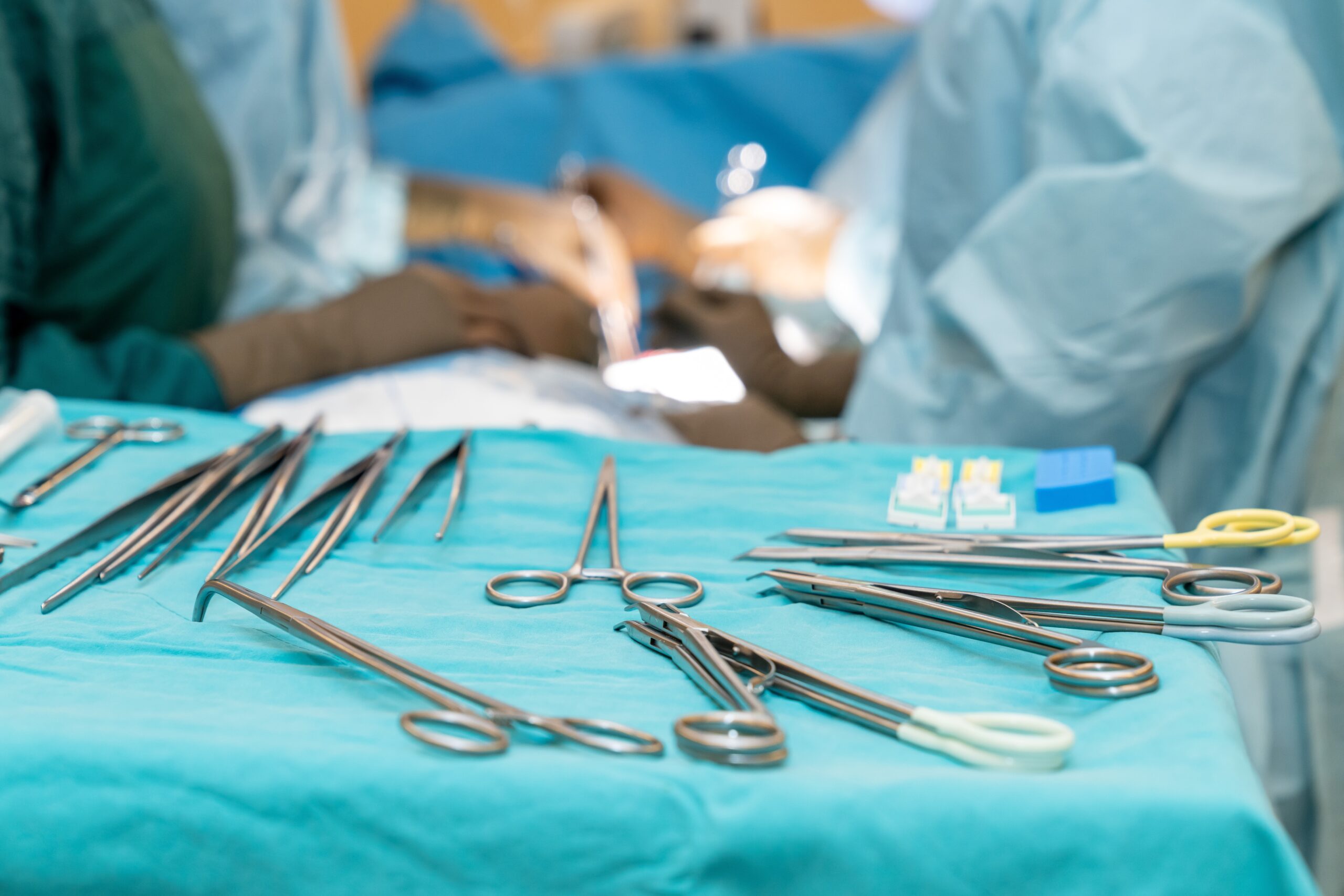
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note AI systems are only as secure and reliable as the data that powers them. That’s the central message of a guidance sheet jointly issued May 22 by the NSA, CISA, FBI, and cybersecurity agencies from Australia, New Zealand, and the UK. The document outlines best practices for securing…

Editor's Note The Centers for Medicare & Medicaid Services (CMS) has updated hospital price transparency guidelines to require publishing real, calculated dollar amounts rather than placeholders and estimates. As detailed in the agency’s May 22 announcement, the revision complies with President Trump’s February 25 Executive Order 14221, “Making America Healthy…

Editor's Note The American Hospital Association (AHA) has called on the Department of Health and Human Services (HHS) to eliminate or ease a variety of federal regulations, arguing that excessive administrative rules drive up costs, reduce patient access, and hinder innovation, Modern Healthcare reported May 13. According to the article,…