
Editor's Note Federal regulation of hospital mergers is inadequate, according to an April antitrust enforcement study scheduled to be published by the American Economic Association. According to a June 14 report in Modern Healthcare, researchers at universities including Harvard and Yale analyzed insurance claims data from Aetna, Humana, and UnitedHealthcare,…
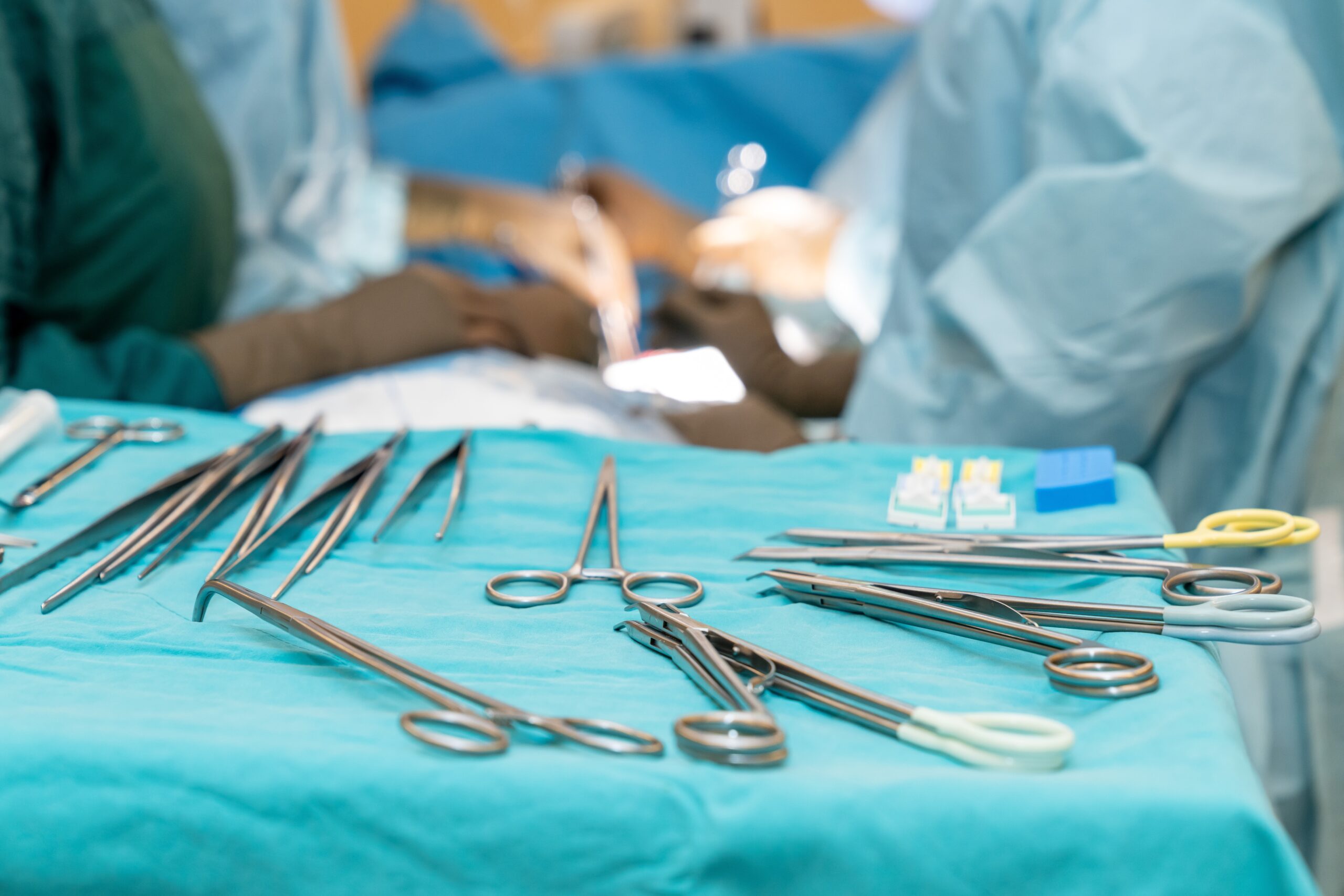
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has updated its standard on ethylene oxide (EO) sterilizers for healthcare facilities, according to a June 12 press release. The first update in two decades, the fourth edition ANSI/AAMI ST24:2024 covers labeling, safety, performance, and testing requirements for general-purpose…

Editor's Note The state agency overseeing Oregon’s hospitals has received a “flood of complaints” due to a “first-of-its-kind” law mandating progressively stricter nurse and certified nursing assistant (CNA) staffing ratios, according to a June 7 report in KMTR. Passed after extensive negotiations among hospital executives, staff, and nurse unions…

Editor's Note New standards from The Environmental Protection Agency promise to cut nationwide emissions of ethylene oxide—employed to sterilize more than half of US medical devices—by more than 90 percent. According to a March 15 MedPage Today report, the aim is to reduce cancer risk among the 13 to 14…
Editor's Note The FDA on March 15 issued its third warning letter to endoscope manufacturer Olympus Medical, following a manufacturing site inspection and 160 complaints of device failure, as reported by Beckers. The warning letter comes after a Tokyo, Japan facility inspection, where the Single-Use Distal Covers for Duodenoscopes and…
Editor's Note The Centers for Medicare and Medicaid Services (CMS) on January 26 announced that 764 hospitals will face payment cuts in FY 2022 under its Hospital-Acquired Condition (HAC) Reduction Program, the January 31 Advisory Board reports. Hawaii and Idaho were the only states whose hospitals did not receive penalties.…
Editor's Note On January 19, a federal court dismissed a lawsuit filed by the state of Texas opposing the federal vaccine mandate. This came just days after the Supreme Court also ruled to uphold the mandate “to promote and protect patient health and safety.” On January 20, CMS issued updated…
Editor's Note The National Institute for Occupational Safety and Health (NIOSH) has honored a request by N95 respirator mask manufacturer, ALG Health (Bryan, Ohio), to rescind all respirator approvals issued to ALG Health, effective immediately. As of January 6, any of the following ALG Health N95 respirators marked with a…

Health systems across the country are enacting COVID-19 mandates to comply with a broad-sweeping federal requirement. Amid reports of high compliance rates, the mandate has triggered passionate and visceral responses on both sides of the debate. While some healthcare employees are eager to roll up their sleeves, others decry the…