
Although I am not a healthcare professional, working for OR Manager offers a peek behind the curtain. What I have learned so far has left me feeling a bit conflicted. When I took this job back in December, I assumed the hospital ecosystem was driven entirely by the Hippocratic Oath.…
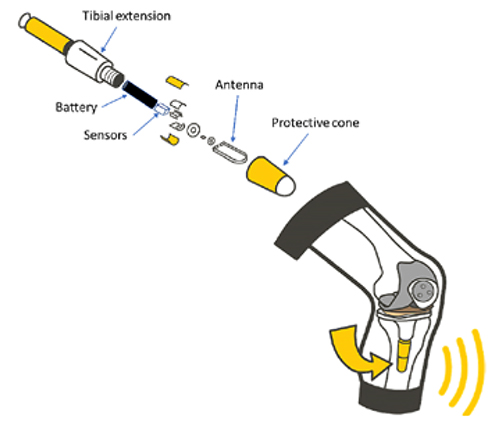
Takeaways Transmitting health metrics directly from a surgical implant reduces the need for in-person followup and offers more objective data on recovery than patient-reported measures. This technology’s potential extends beyond knees. Whatever the nature of the treatment, postop monitoring is critical. Patient education is essential for setting up the data…

The administrator of an ambulatory surgery center (ASC) wears many hats, doing every odd job in the book to keep their free-standing center safe, compliant, and operational. But what does “wearing many hats” mean exactly? OR Manager spoke with Nyleen Flores, CPMSM, CPCS, CPCO, CASC, chief administrative officer at Lake…

Some 800,000 knee replacements and 550,000 hip replacements are performed in the US each year. Factoring in the ever-expanding aging population, projections show the figure for knee replacements alone will explode to 3.5 million procedures being done annually by 2030—and that is just one type of procedure within a single…

Editor's Note Many youths continue to take opioids months after undergoing surgery, according to a recent multi-institutional study published in JAMA Network. Medical Xpress covered the news July 11. Conducted by researchers from CHOP, Massachusetts General Hospital, University of Pennsylvania, and Stanford Medicine, the study found 1 in 6 youths…

Editor's Note An increasing number of young and middle-aged adults are opting for knee and hip replacements earlier in life, breaking the stereotype that such surgeries are primarily for seniors aged 65 or older. That is according to a July 24 article in U.S. News & World Report. Citing research…

Editor's Note Intraoperative infusion of dexmedetomidine (DEX) could help improve glycemic control and reduce insulin requirements in diabetic patients undergoing cardiac surgery, according to a July 25 article in Medical Dialogues. The article focuses on a prospective observational study published in the journal Annals of Cardiac Anesthesia. The study included…

Editor's Note Healthcare providers should consider strategies to conserve BD BACTEC blood culture media bottles for patients at highest risk due to an ongoing shortage, according to a July 10 report from The US Food and Drug Administration (FDA). Attributing the shortage to supplier issues, FDA warns that the disruption…

Editor's Note North Korean hackers targeted U.S. hospitals and healthcare systems with ransomware to fund a covert information exfiltration campaign against American military and scientific entities, according to a July 25 report from CBS News. The first attack was a May 2021 ransomware infiltration of a hospital in Kansas. The…

Editor's Note A needle-based technique that blocks sensory and motor function below the chest without intubation or general anesthesia makes surgery safer for pediatric patients, according to a July 15 report in Michigan Medicine. The University of Michigan's pediatric spinal anesthesia program, also implemented at University of Michigan-Sparrow Health Center,…