
Editor’s Note: This page is a companion piece to the main article, Proper planning prevents OR construction, renovation cost creep. It brings together four focused updates on OR and ambulatory surgery center (ASC) construction and renovation: Regulatory shifts: The Joint Commission's updated safety and sustainability requirements for hospitals and ASCs, including new…
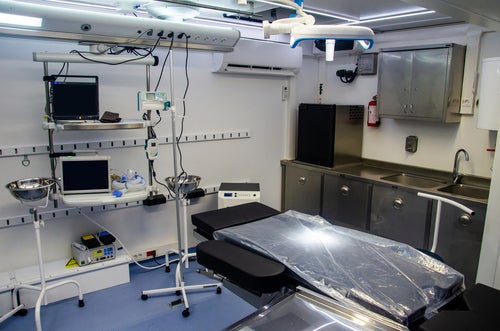
Imagine an innovative, safe, and highly efficient OR not confined by walls but on wheels—crossing rugged terrains, bustling cities, and disaster-stricken areas to deliver life-saving surgical care in underserved areas. That is the premise and promise of mobile ORs. They are not just mobile units. With some of the technological…

Editor's Note The Joint Commission has launched a major redesign of its healthcare accreditation and certification programs with Accreditation 360: The New Standard. According to a June 30 announcement, the new framework introduces outcome-focused performance tools, eliminates hundreds of requirements, and promises to made standards publicly accessible. Reportedly supported by…

Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…

Editor's Note Failure to document thoroughly, position patients safely, and follow facility policies are leading causes of malpractice claims against perioperative nurses, according to an analysis published in the AORN Journal on May 28. Although physicians are more frequently named in malpractice suits, nurses are the primary provider responsible in…

Editor's Note The Joint Commission and the Coalition for Health AI (CHAI) are partnering to set national standards for responsible artificial intelligence (AI) use in healthcare, according to a June 11 announcement from the organizations. This effort will deliver AI implementation guidance, tools, and a certification program to over 80%…

Editor's Note The US Government Accountability Office (GAO) is urging the Department of Health and Human Services (HHS) to strengthen its approach to diagnostic testing during a pandemic or other public health crisis, citing ongoing gaps in leadership, coordination, and readiness, according to a June 5 report from the Center…
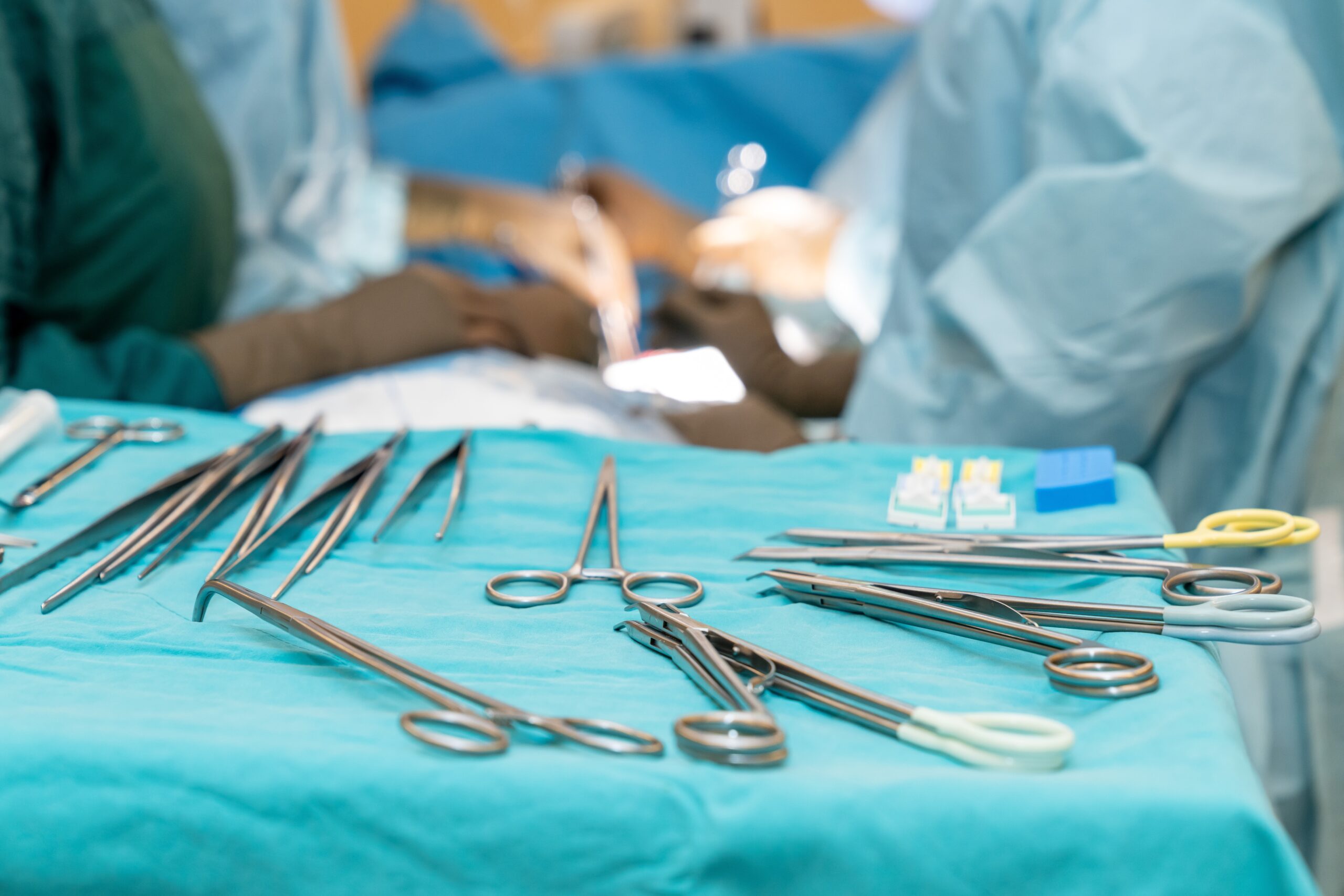
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note Postoperative nausea and vomiting (PONV) remain a significant concern in thyroidectomy patients, with incidence rates reaching up to 80% in high-risk groups, Medical Dialogues April 25 reports. Despite effective intraoperative and postanesthesia care unit (PACU) protocols, gaps often arise during patient transitions to wards or intensive care units,…
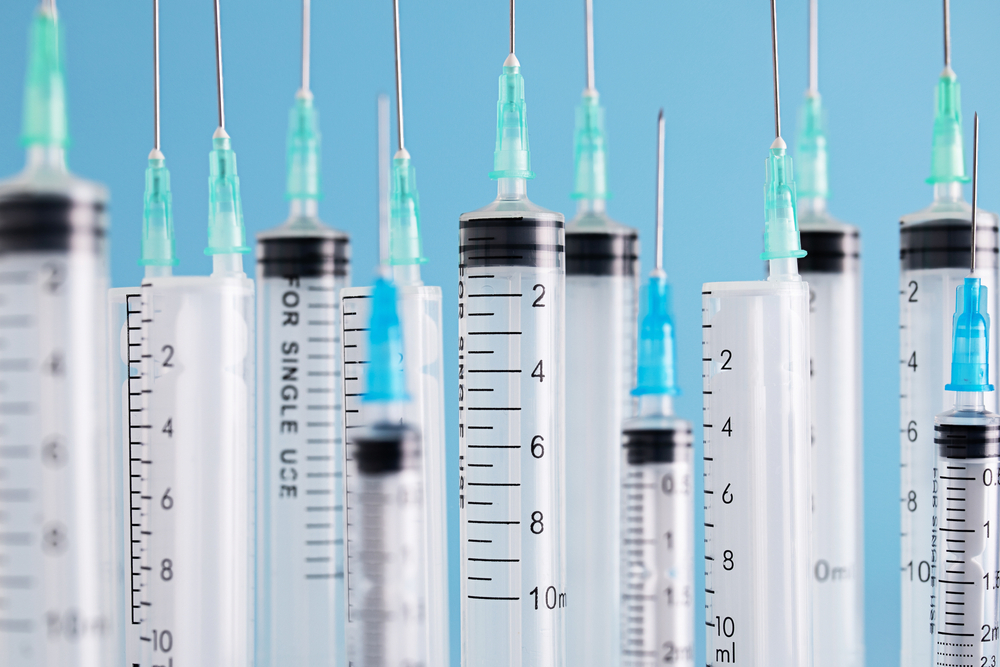
Takeaways • The prevalence of needlesticks and other sharp object injuries to OR team members is 42.8%, an increase of 16% over the past decade. • New research and perspectives are shaping the discourse around sharps safety, such as new and expanded evidence-based practices presented in AORN’s 2025 update to…