
The centralization of medical device processing to one facility is becoming more prevalent. Centralizing sterile processing activities reduces expenses while concentrating expertise. However, this also introduces new concerns. When sterile processing is located within the same building where instrumentation is used, transport occurs over smooth floors in a controlled environment…

Editor's Note Conventional cleaning protocols fail to remove visible soil and debris from lumened surgical instruments, raising urgent concerns about patient safety and sterilization efficacy. That’s the central finding of a study published February 11 in The American Journal of Infection Control, which used borescopes to inspect the lumens of…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…

For many in the healthcare industry, imagining surgery without onsite sterile processing seems unthinkable. Then again, performing total joints in an ambulatory surgery center (ASC) was unthinkable 10 years ago. ASC sterile processing departments (SPDs) are generally not designed to handle the high volumes of instrument trays, vendor trays, and…
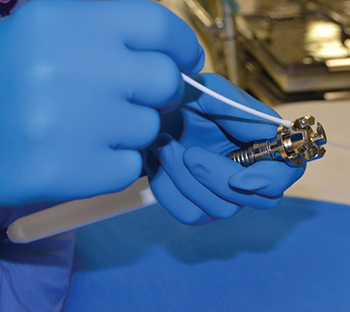
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…

Editor's Note A new guidance document covering the entire process for the selection, labeling, and sterile processing of dilators and ultrasound probes is available from The Association for the Advancement of Medical Instrumentation (AAMI). Released April 17, AAMI TIR99:2024; Processing Of Dilators, Transesophageal And Ultrasound Probes In Health Care Facilities…

Editor's Note New standards from The Environmental Protection Agency promise to cut nationwide emissions of ethylene oxide—employed to sterilize more than half of US medical devices—by more than 90 percent. According to a March 15 MedPage Today report, the aim is to reduce cancer risk among the 13 to 14…

Editor's Note Compared with standard wound dressings, single-use negative pressure wound therapy (NPWT) devices can reduce the incidence of surgical site infection (SSI) in at-risk patients with closed surgical incisions across a range of surgical specialties, according to a data review highlighted in the February issue of the American Journal…

Sterile Processing Department (SPD) managers and technicians know a thing or two about pressure. In a recent webinar covering sterile processing basics, Cori L. Ofstead, MSPH, president and CEO of Ofstead & Associates, Inc, and Abby Smart, MPH, research associate, cited the example of a 480-bed hospital that performed 13,650…

Immediate use steam sterilization (IUSS, a standard steam sterilization cycle with little to no dry time) is considered safe for patient care when the processes recommended in Association for the Advancement of Medical Instrumentation (AAMI) standards and AORN guidelines are followed. IUSS is a valuable option in an emergency. Lack…