
Editor's Note Rebuilding trust and redefining teamwork between the OR and sterile processing department (SPD) turned a near-failure into a high-functioning system at UCLA Health, according to Ronald Perez, JD, MSN, RN, NEA-BC, CNOR, executive director of perioperative services, and Jasmine Briones, MSN, RN, CNOR, director of perioperative services. What…

Editor's Note The Food and Drug Administration (FDA) has announced three new recalls between October 14 and 16 that may affect OR inventory and perioperative workflows across multiple service lines. Each recall targets a commonly used product in surgical or imaging settings, prompting leaders to review supplies and coordinate with…

Editor's Note NDM-producing carbapenem-resistant Enterobacterales are climbing fast and straining treatment choices, according to a September 23 release from the Centers for Disease Control and Prevention (CDC) and published in the Annals of Internal Medicine. The agency warns NDM-CRE infections rose more than 460% in the US from 2019 to…
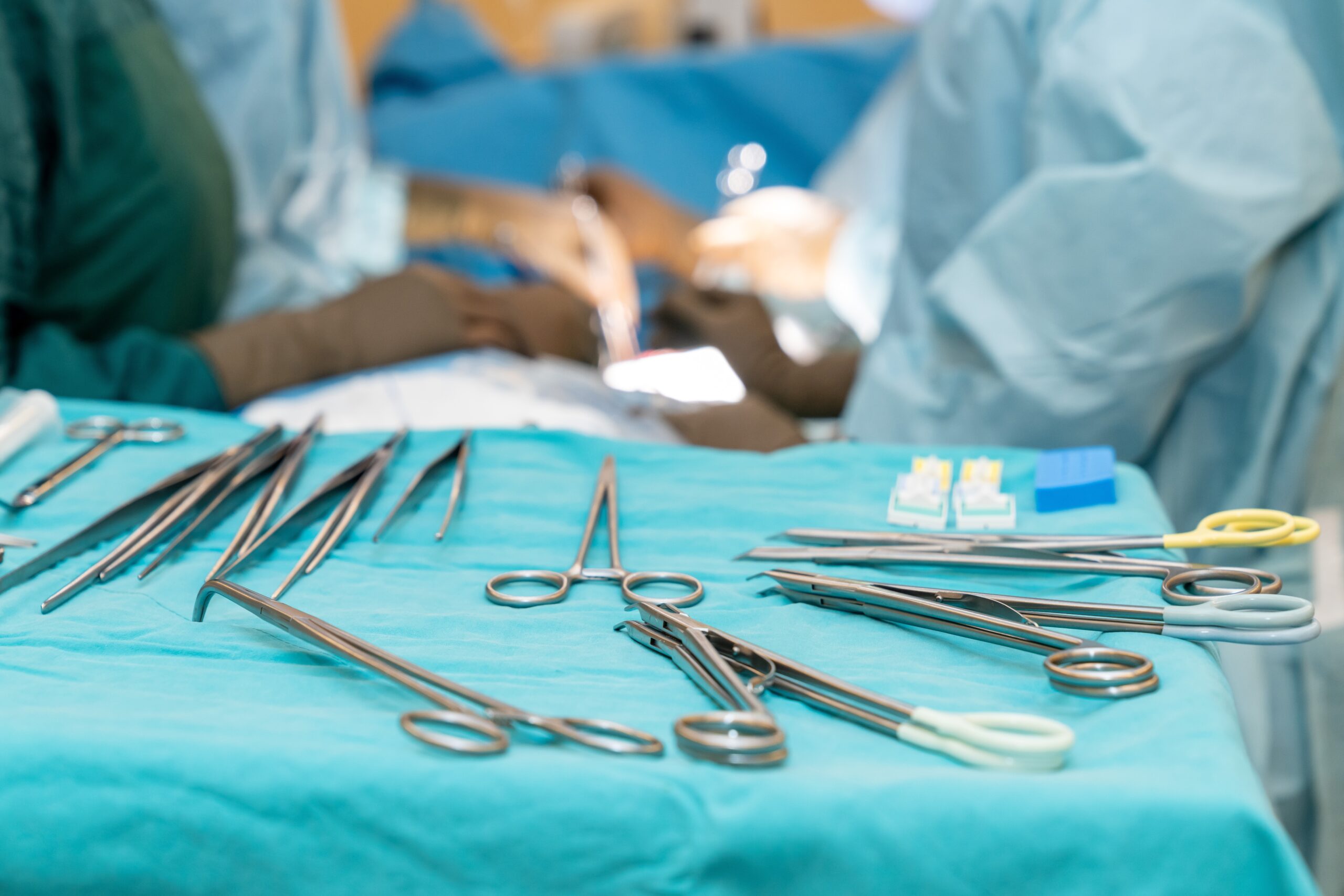
Once limited to hospital inpatient settings, total joint surgery is increasingly common at ambulatory surgery centers (ASCs) across the US. What is not so common is performing these complex procedures without the benefit of an onsite sterile processing department (SPD). And yet, that is exactly what we have accomplished at…

Editor's Note AI-enabled sensors, smart surveillance systems, and predictive analytics are advancing perioperative workflows while helping prevent breaches that can lead to surgical site infections (SSIs). This is the primary takeaway of a July 15 Q&A with Herman DeBoard, PhD, CEO of Huvr Inc., in Infection Control Today. As detailed…

Editor's Note A study published July 9 in the American Journal of Infection Control found that clean paper towels are as effective—and in some cases more efficient—than sterile alternatives for surgical hand antisepsis. The results support their use as a cost-saving and safe alternative to sterile hand-drying products in surgical…

Editor's Note Researchers studying the exposure of sterile surgical slush to open air urge the adoption of closed-system technology to alleviate risks to sterility and surgical outcomes, according to a May 19 article in OR today. The article focuses on a time and motion study led by perioperative nursing leaders…

Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note Although health system layoffs so far involve mostly non-clinical staff, the cuts nonetheless threaten both hospital operations and patient safety, according to a May 21 article in Modern Healthcare. As detailed in the article, thousands of nonclinical workers—such as those working in nutrition, janitorial, and sterile processing—have been…

Editor's Note Air quality in cardiac ORs may be a silent driver of surgical site infections (SSIs), with airborne contamination linked to significantly elevated infection risk and mortality—especially when ventilation is suboptimal. A newly published study covered by Medical Dialogues May 19 reveals that one-third of bacteria in cardiac procedures…