CBS affiliate KMOV-TV broadcast an investigation last week that reported concerning conditions about the state of surgical instruments at Barnes-Jewish Hospital in St. Louis. The TV station said it received tips from anonymous whistleblowers who “are terrified of losing their jobs but felt compelled to speak out” about “potentially contaminated…
Editor's Note Updates to evidence-based guidance in the AORN 2026 Guideline for the Care and Cleaning of Surgical Instruments were summarized in a November 7 AORN news story to outline key updates such as new PPE recommendations for perioperative staff working in the decontamination area. For example, new recommendations in…

Editor's Note Rebuilding trust and redefining teamwork between the OR and sterile processing department (SPD) turned a near-failure into a high-functioning system at UCLA Health, according to Ronald Perez, JD, MSN, RN, NEA-BC, CNOR, executive director of perioperative services, and Jasmine Briones, MSN, RN, CNOR, director of perioperative services. What…

Editor's Note Introducing preassembled surgical trays sharply reduces OR waste and setup time while improving staff workflow satisfaction, Surgeries October 8 reports. The prospective study, conducted in a high-volume German urology center, compared tray-based setups with the “standard approach” for preparation across 64 procedures and found measurable ecological and operational…

Editor's Note University of California San Francisco (UCSF) became the first university to certify medical students as robotic surgery bedside assistants, giving future surgeons unprecedented hands-on training in a rapidly growing field, an August 25 UCSF news release reports. The program reportedly positions learners alongside attending surgeons, nurses, and scrub…

Editor's Note The US Food and Drug Administration (FDA) has designated the recall of Ethicon Endo-Surgery, LLC’s Endopath Echelon Vascular White Reload for Advanced Placement Tip a Class I, the most severe category indicating risk of serious injury or death. As detailed in the agency’s July 25 announcement, the recall…
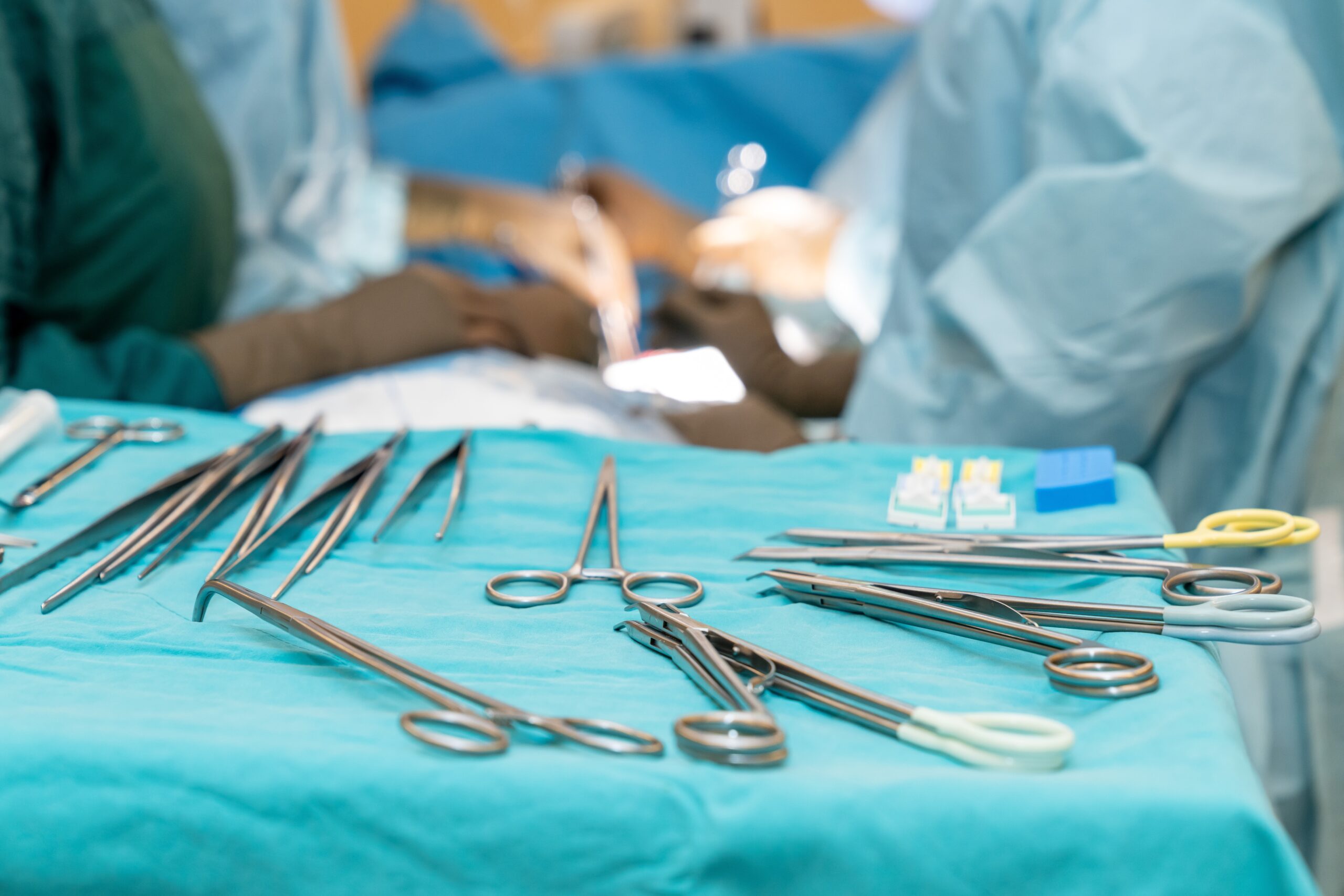
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note A physician-led sustainability initiative focused on waste reduction and eliminating desflurane an nitrous oxide is paying off for Keck Medicine of the University of Southern California (USC), according to a May 19 report in USC Trojan Family Magazine. The article, part of a series focused on waste…

In the OR, precision and focus can mean the difference between life and death. However, surgical patient outcomes hinge on more than the competence of those working in these inherently intense environments. Every procedure also depends on the laborious, behind-the-scenes efforts of the people responsible for ensuring every surgical instrument…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…