
For many in the healthcare industry, imagining surgery without onsite sterile processing seems unthinkable. Then again, performing total joints in an ambulatory surgery center (ASC) was unthinkable 10 years ago. ASC sterile processing departments (SPDs) are generally not designed to handle the high volumes of instrument trays, vendor trays, and…

Editor's Note Using a device they call a “space hairdryer,” researchers in Austria applied gentle shockwaves to regenerate heart tissue after coronary artery bypass graft surgery (CABG) in a study with potential implications for millions of patients, BBC News reported June 20. Researchers are now seeking larger trials, European regulatory…
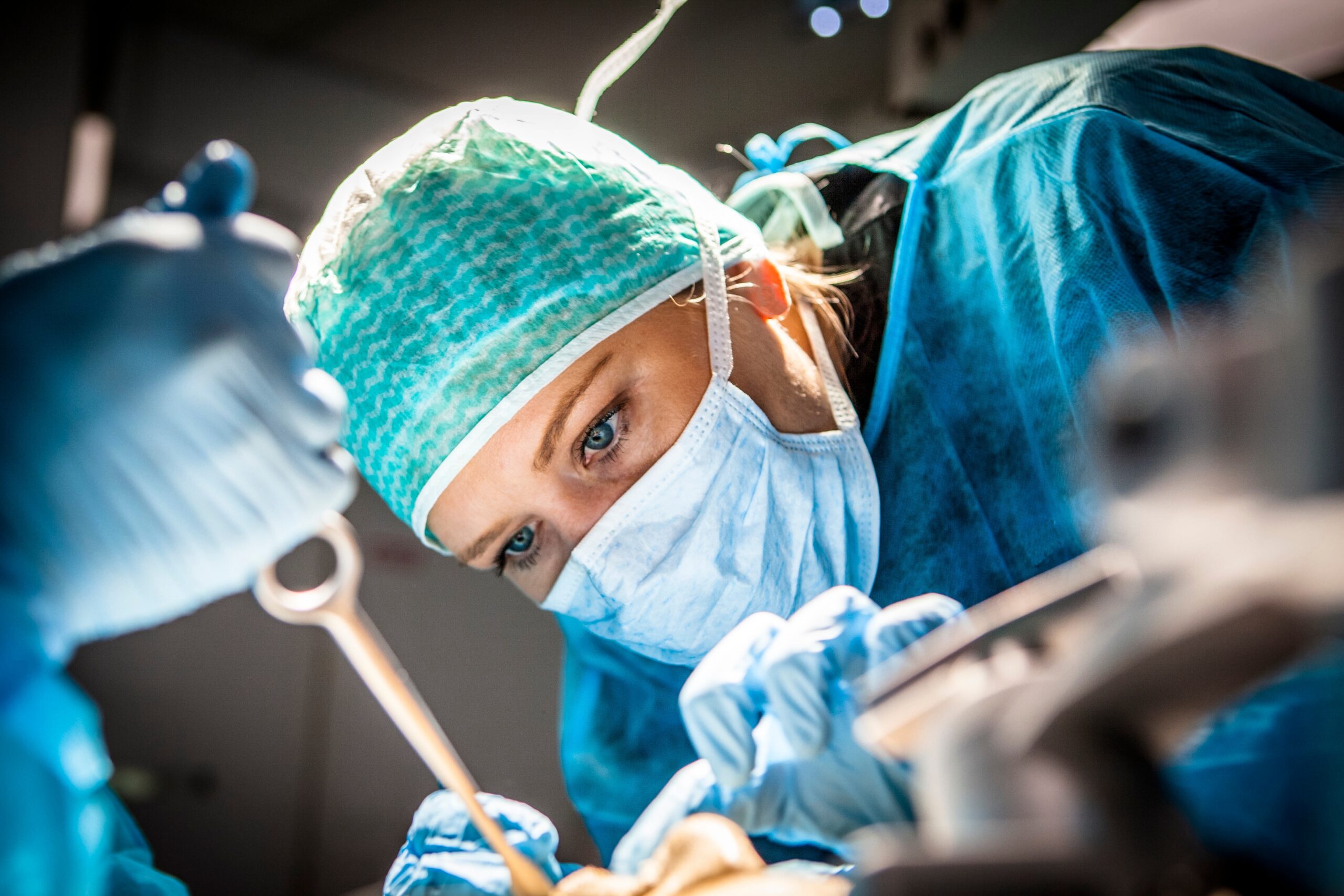
Takeaways Although women comprise half the population, they were left out of medical research on major causes of death for both women and men—cancer, heart disease, and stroke—until 1990. Using surgical tools designed by men, for men can impact every aspect of a woman surgeon’s work, from learning new procedures…
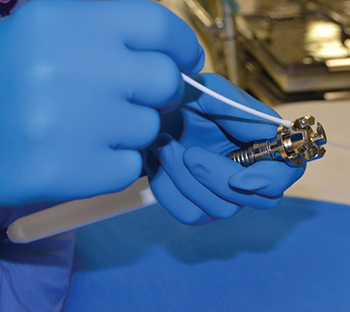
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…

Editor's Note A patient undergoing emergency neurosurgery at Northwestern Medicine became the first to benefit from a neurosurgical drill designed to eliminate the need for hand-crank operation. According to a March 5 report from Northwestern, the procedure occurred in October at Northwestern Memorial Hospital, “when Northwestern Medicine neurosurgeon Matthew Potts, MD used…

Sterile Processing Department (SPD) managers and technicians know a thing or two about pressure. In a recent webinar covering sterile processing basics, Cori L. Ofstead, MSPH, president and CEO of Ofstead & Associates, Inc, and Abby Smart, MPH, research associate, cited the example of a 480-bed hospital that performed 13,650…

Immediate use steam sterilization (IUSS, a standard steam sterilization cycle with little to no dry time) is considered safe for patient care when the processes recommended in Association for the Advancement of Medical Instrumentation (AAMI) standards and AORN guidelines are followed. IUSS is a valuable option in an emergency. Lack…
Editor's Note Researchers for a long time have expressed concern about how time and temperature might contribute to changes that affect the cleaning and sterilization of instruments. However, there have been few studies examining these claims. A September 2023 study in Biomedical Instrumentation and Technology from the Association for the…
Editor's Note In a June 27 letter, the American Hospital Association (AHA) urged the Environmental Protection Agency (EPA) “to avoid disrupting healthcare delivery through the unintentional fracturing” of the medical device supply chain with its proposed ethylene oxide (EtO) standards. The EPA proposes: reducing EtO emissions by 80% adding new…

Many healthcare professionals are aware of single-use device reprocessing and its benefits. According to the Association of Medical Device Reprocessors, the savings generated by such programs can strengthen a facility’s financial sustainability and help provide a path for more responsible environmental stewardship. Sometimes, ambulatory surgery centers (ASCs) may experience situations…