
Editor's Note The US Department of Health and Human Services (HHS) has withdrawn its appeal against a federal court decision that blocked a rule restricting hospitals from using tracking technology on their websites, HealthLeaders August 30 reports. This rule, proposed in December 2022 by the HHS Office for Civil Rights,…

Asking who, what, why, when, where, and how—otherwise known as the “5 Ws and an H”— is a time-tested way for writers and researchers to ensure comprehensive coverage of any topic. Here, we apply this framework from the perspective of sterile processing department (SPD) professionals seeking to start a water…

There is no shortage of advice, opinions, and proposed solutions when it comes to staff shortages, but the issue continues to plague healthcare systems nonetheless. For a couple of years now, speakers at the OR Business Management Conference and OR Manager Conference have been asking attendees, “Who still struggles to…
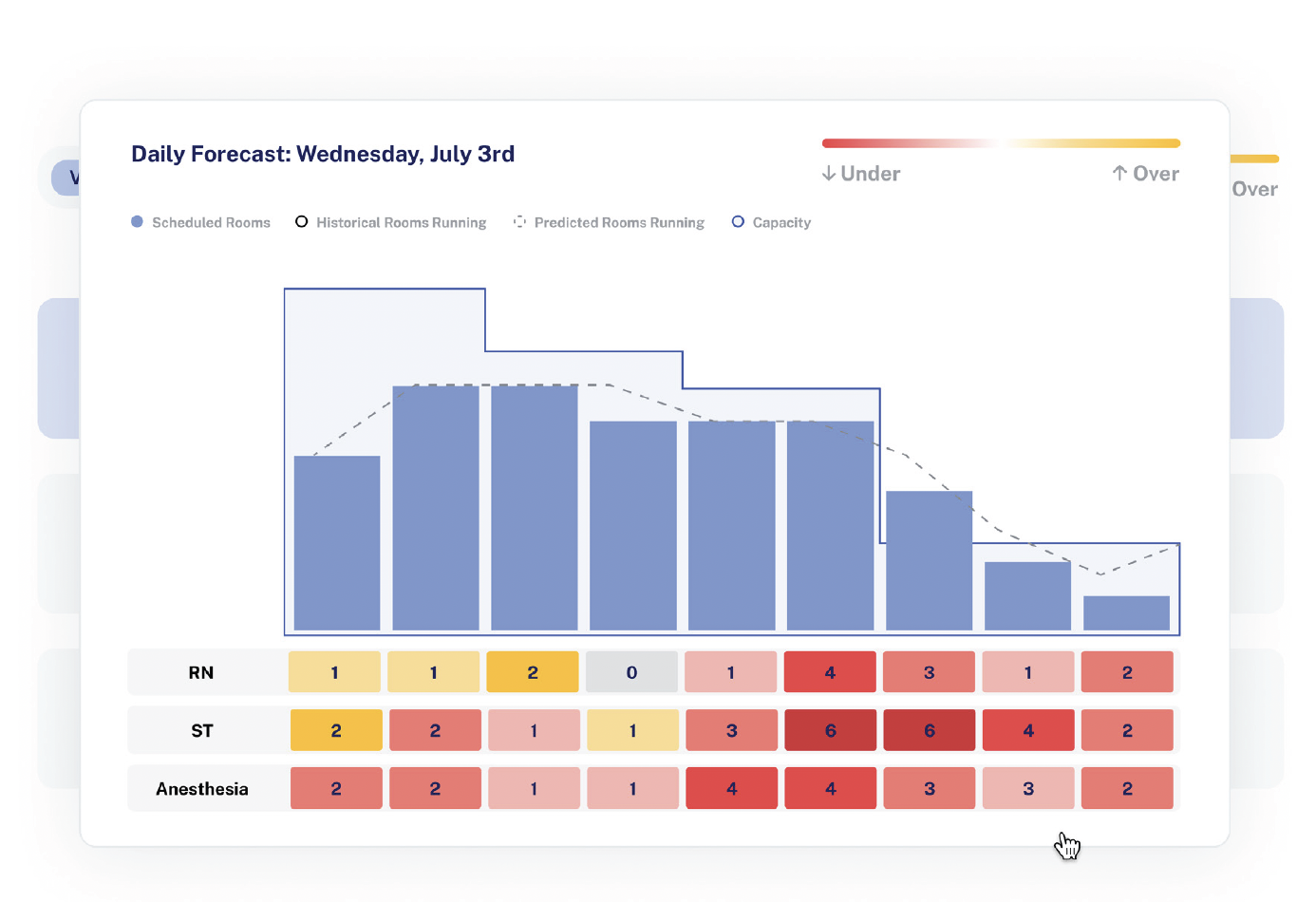
Perioperative leaders face mounting pressures to optimize resources, reduce costs, and improve patient outcomes. However, one challenge stands out among the rest: OR staffing shortages. According to a November/December survey conducted by LeanTaaS in collaboration with OR Manager, staff recruitment and retention is a top priority for OR leaders this…

Editor's Note The US Food and Drug Administration (FDA) has designated Defibtech, LLC’s recall of RMU-2000 ARM XR Chest Compression Devices as Class 1, the most severe category indicating serious risk of injury or death. A motor issue could stop compressions in adults whose hearts suddenly stop, according to the…

Editor's Note Recent research sheds new light on addressing two of the most pressing problems for surgical care: handoff communication failures and care bias and inequities leading to adverse—and preventable—events. These problems are the subjects of two separate success stories in the August issue of The Joint Commission Journal…

Editor's Note The US Food & Drug Administration (FDA) has approved immunotherapy durvalumab for perioperative treatment of resectable non-small cell lung cancer (NSCLC), according to an August 15 announcement. The approval is for durvalumab (Imfinzi, AstraZeneca) in combination with platinum-based chemotherapy as a neoadjuvant treatment, followed by durvalumab alone as…

Editor's Note The US Food and Drug Adminstration (FDA) has classified a recall of ICU Medical’s Plum 360, Plum A+ and Plum A+3 infusion pumps as Class 1, the most severe category indicating risk of serious injury or death. According to he agency’s August 20 announcement, the company is updating…

Editor's Note A newly developed biomaterial could treat crippling arthritis by prompting the growth of new cartilage, according to an animal study conducted at Northwestern University and published in the Proceedings of the National Academy of Sciences. According to an August 6 article by U.S. News and World Report, the…

Editor's Note An August 6 report in MedPage Today details how the Physicians Committee for Responsible Medicine (PCRM) is pushing to end the practice of using live animals for physiology training. According to the article, some surgical residencies use live animals (usually pigs) as practice patients. In contrast, only 3%…