
Editor's Note Hospitals and health systems have largely returned to normal operations in the wake of a global IT outage caused by a faulty update from cybersecurity company CrowdStrike, according to a July 29 article in Becker’s Health IT. The outage, which began July 18, resulted in many health systems'…

Although I am not a healthcare professional, working for OR Manager offers a peek behind the curtain. What I have learned so far has left me feeling a bit conflicted. When I took this job back in December, I assumed the hospital ecosystem was driven entirely by the Hippocratic Oath.…

One privilege of living at this time in history is the availability of choice, especially for health and surgical needs. Those who make healthcare their business understand this as well. According to Tanna et al, ambulatory surgery centers (ASCs) are spreading to rural areas, hospitals are creating hybrid outpatient surgery…
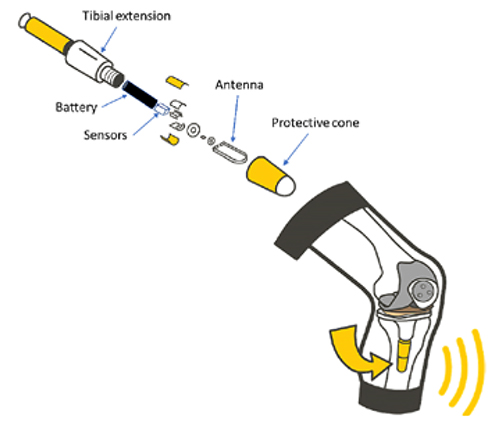
Takeaways Transmitting health metrics directly from a surgical implant reduces the need for in-person followup and offers more objective data on recovery than patient-reported measures. This technology’s potential extends beyond knees. Whatever the nature of the treatment, postop monitoring is critical. Patient education is essential for setting up the data…

I still recall being handed roughly 10 boxes of suture anchors to practice with years ago at New York University (NYU) medical school. “Aren’t these needed here?” I asked incredulously. “Not at all,” came the answer, despite a hefty price tag of $1,000 per box. In fact, getting the suture…

The administrator of an ambulatory surgery center (ASC) wears many hats, doing every odd job in the book to keep their free-standing center safe, compliant, and operational. But what does “wearing many hats” mean exactly? OR Manager spoke with Nyleen Flores, CPMSM, CPCS, CPCO, CASC, chief administrative officer at Lake…

Editor's Note An increasing number of young and middle-aged adults are opting for knee and hip replacements earlier in life, breaking the stereotype that such surgeries are primarily for seniors aged 65 or older. That is according to a July 24 article in U.S. News & World Report. Citing research…

Editor's Note Intraoperative infusion of dexmedetomidine (DEX) could help improve glycemic control and reduce insulin requirements in diabetic patients undergoing cardiac surgery, according to a July 25 article in Medical Dialogues. The article focuses on a prospective observational study published in the journal Annals of Cardiac Anesthesia. The study included…

Editor's Note Healthcare providers should consider strategies to conserve BD BACTEC blood culture media bottles for patients at highest risk due to an ongoing shortage, according to a July 10 report from The US Food and Drug Administration (FDA). Attributing the shortage to supplier issues, FDA warns that the disruption…

Editor's Note The medical 3D printing market is expected to double from $2 billion in 2022 to $4 billion by 2026, driven by customization, lower costs, and quick turnarounds, according to analysis from GlobalData. In a July 24 report on the analysis, Medical Device Network outlined this growth as well…