Editor's Note Retooling paper-based measures to electronic format for reporting performance measures can help reduce hospitals’ reporting burden. However, in this study by Joint Commission and State University of New York researchers, a simplified risk model using electronic health record (EHR) elements could not capture most risk factors in the…
Editor's Note The Centers for Medicare & Medicaid Services (CMS) on June 21 issued final updates to its national coverage policy for Transcatheter Aortic Valve Replacement (TAVR). The update provides more flexibility in how centers meet volume requirements for performing TAVR, while emphasizing the importance of the heart team for…
Editor's Note A visible-light continuous environmental disinfection (CED) system, used with manual cleaning, resulted in a significant reduction in microbial surface contamination and surgical site infections (SSIs) in an orthopedic OR, in this study. Samples were taken from 25 surfaces within two contiguous ORs sharing an air supply after manual…
Editor's Note At a precision medicine conference in Boston on June 18, Harvard Law School professor Jonathan Zittrain likened the use of artificial intelligence (AI) in healthcare to asbestos, saying: “it’s all over the place, even though at no point did you explicitly install it, and it has possibly some…
Editor's Note The Joint Commission on June 19 announced that the National Association for Healthcare Quality (NAHQ) has issued a new report−“Key Workforce Competencies for Quality-Driven Healthcare”− that focuses on creating a new framework for competencies needed by healthcare organizations to meet new goals for quality-driven healthcare. Among the competencies:…
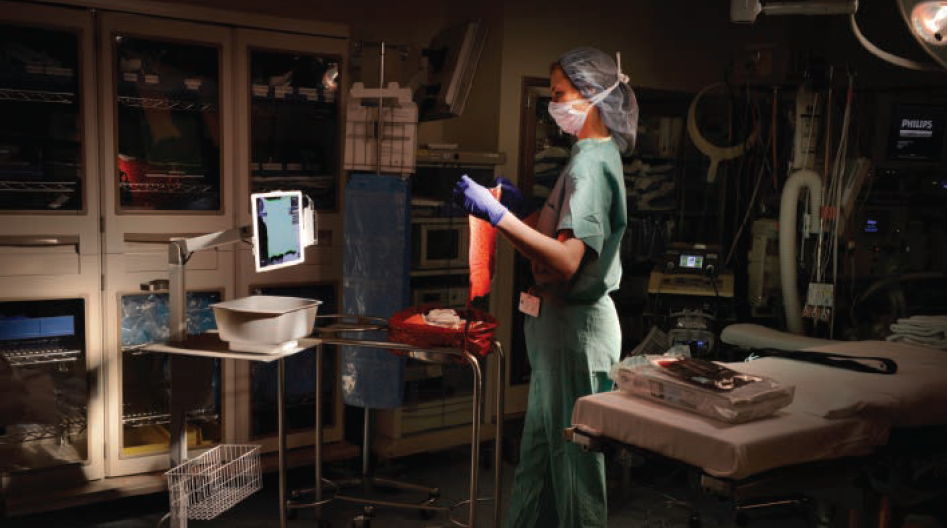
Blood loss during labor and delivery (L&D) and surgical procedures can lead to serious complications that might be prevented with early detection; however, detection can be challenging. For example, clinicians have traditionally estimated blood loss visually—a subjective and often inaccurate process. Humans’ eyes simply aren’t good at making precise measurements,…
OR leaders spend much of their day ensuring the surgery schedule runs smoothly, a task that depends on the teams within each of the ORs. Highly functioning teams produce optimal results, including good patient outcomes, satisfied surgeons, and efficient use of resources. But developing a highly functional team with good…
Editor's Note A new report from the National Institutes of Health, Radiological Society of North America, American College of Radiology, and The Academy, provides a roadmap for translational research on artificial intelligence (AI) in medical imaging. The report summarizes key priorities: creating structured AI use cases that define and highlight…
Editor's Note Stryker announced on May 20 that the Food & Drug Administration (FDA) granted premarket approval for its Neuroform Atlas stent system for intracranial aneurysms, the May 20 MassDevice reports. The self-expanding nitinol stent can now be used with neurovascular embolization coils to treat patients with saccular wide-necked…
Editor's Note The Food and Drug Administration (FDA) on May 21 released a letter to healthcare professionals about the most recent, interim post-approval study (PAS) results for the Abiomed Impella RP System, a percutaneous heart pump for right heart support. The latest interim results show a lower survival rate…