
Editor's Note Cardiac surgical team members recognize distinct critical time points during cardiac surgery, but a high degree of variability exists between members as to the importance of these times, which suggests an absence of a shared mental model, this study finds. Cardiac team members from three institutions developed a…

Editor's Note Intraoperative adverse events are independently associated with increased postoperative mortality, morbidity, and prolonged length of stay (LOS), this study finds. Of 9,288 abdominal surgical procedures analyzed, 183 had intraoperative adverse events. Most consisted of bowel (44%) or vessel (29%) injuries, which were addressed intraoperatively (92%). Multivariate analysis showed…
Healthcare is striving to become an industry of high-reliability organizations, and part of being a high-reliability industry means staying vigilant and identifying problems proactively. ECRI Institute’s annual Top 10 list helps organizations identify looming patient safety challenges and offers suggestions and resources for addressing them. ECRI Institute relied on event…

Editor's Note Adverse event-free admissions provide a patient-centered indicator that aligns directly with patient safety, this study finds. Using Medicare data from 2009 to 2011, researchers found that 64% of 24 million admissions had no adverse events. Multiple events were recorded in 22.7%, and 15% of these had more than…
Editor's Note The Food and Drug Administration (FDA) on March 18 issued a Safety Alert for Abbott Vascular’s (Santa Clara, California) Absorb GT1 Bioresorbable Vascular Scaffold (BVS). The alert was issued to inform healthcare providers of an increased rate of major adverse cardiac events in patients receiving the BVS, when…
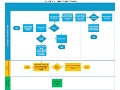
Editor's Note In this study, an automated harm trigger system developed by the Adventist Health System Patient Safety Organization (Altamonte Springs, Florida) enabled the identification of patients who may have been harmed or at risk for harm. Nurse reviewers analyzed electronic health records of current patients with positive triggers to…

Editor's Note In this advisory, the Pennsylvania Patient Safety Authority reviews retained surgical item (RSI) events in Pennsylvania hospitals and RSI guidelines from various organizations. Analysis of events reported to the Pennsylvania Patient Safety Authority from 2014 to 2015 reveals 112 RSIs that met the definitions of the National Quality…

Editor's Note There were about 3.1 million fewer hospital-acquired conditions (HACs) between 2010 and 2015, according to a newly released report from the Agency for healthcare Research and Quality (AHRQ). Most of the decline was because of a: 42% reduction in adverse drug events 23% drop in pressure ulcers 15%…

Editor's Note In this study from the Massachusetts General Hospital, Boston, major intraoperative adverse events were independently associated with a two-fold increase in readmissions. Of 9,274 surgical procedures analyzed, 921 resulted in readmission. Of these, 183 had confirmed intraoperative adverse events, 73 of which were major events. Procedures with major…
Editor's Note The Joint Commission announced February 22 that it had updated its sentinel event statistics through the end of 2016. Of 824 events reviewed, unintended retention of a foreign object topped the list at 120. Wrong-patient, wrong-site, or wrong-procedure was second at 104 events, and operative/postoperative complication was seventh…