Editor's Note The Food and Drug Administration (FDA) on June 16 announced the recall by Alvogen/Hospira Inc, a Pfizer company, of seven lots of Clindamycin Injection USP ADD-Vantage Vials. The recall was initiated because microbial growth was detected during a routine simulation of the manufacturing process, which represents the potential…
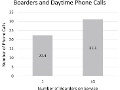
Editor's Note Surgical ICU patients boarding in alternative ICUs because of overcrowding are often seen at the end of rounds, receive fewer face-to-face assessments from physicians, and are given less bedside attention by ICU provider teams, this study finds. The researchers found that: caregivers spent about 16% less time on…

Editor's Note The global ambulatory services market was valued at $2.3 trillion in 2016 and is forecast to grow to $3.7 trillion by 2024, according to a new study by Ameri Research. In 2016, primary care offices accounted for the majority of market share at more than 48% because they…
Editor's Note The Food and Drug Administration (FDA) on June 19 announced the recall by Maquet/Datascope (Fairfield, New Jersey) of its System CS100, CS100i, and CS300 Intra-Aortic Balloon Pumps. The recall also applies to System 98 or System 98XT IABP that was converted to a CS100i or CS 300 IABP.…

Editor's Note Techniques used to clean and sterilize flexible ureteroscopes left behind contamination that included debris, residue, and bacteria, in this study presented June 14 at the 44th Annual Conference of the Association for Professionals in Infection Control. Researchers with Ofstead & Associates (St Paul, Minnesota) sampled 16 ureteroscopes at…

Editor's Note The American College of Rheumatology (ACR) and American Association of Hip and Knee Surgeons (AAHKS) have released a new guideline to help reduce postoperative total hip and knee infections through the perioperative management of antirheumatic medications. Among the recommendations: Discontinuing biologic therapy before surgery in patients with inflammatory…
Editor's Note The Food and Drug Administration (FDA) on June 16 announced the recall by Hospira, Inc, a Pfizer company, of the following: 42 lots of 8.4% Sodium Bicarbonate Injection, USP, 50 mL vials 5 lots of Neut (Sodium Bicarbonate 4% additive solution) 5 mL vials 5 lots of Quelicin…

Editor's Note In this study, Medicare’s Hospital Value-Based Purchasing (HVBP) program was not associated with improvements in quality measures of clinical process or patient experience. HVBP also was not associated with a significant reduction in two of three mortality measures (ie, acute myocardial infarction or heart failure). It was associated…

Editor's Note Revisions to the Life Safety (LS) and Environment of Care (EC) chapters for Behavioral Health Care, Laboratory, Nursing Care Centers, and Office-Based Surgery accreditation programs, based on adoption of the 2012 editions of the National Fire Protection Association’s NFPA 101: Life Safety Code and NFPA 99: Health Care…
Editor's Note The Food and Drug Administration (FDA) on June 15 issued a Safety Alert for frameless stereotaxic navigation systems because of navigational accuracy errors during surgical procedures. Some of these errors have led to patient deaths, serious or life-threatening injuries, and inaccurate, aborted, or prolonged surgical procedures. The FDA…